Suppression of microglial activation with innate lymphoid cells
A technology of lymphoid cells and microglia, applied to medical preparations containing active ingredients, peptide/protein ingredients, allergic diseases, etc., can solve problems such as unclear long-term effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0153] The invention also provides the following non-limiting embodiments.
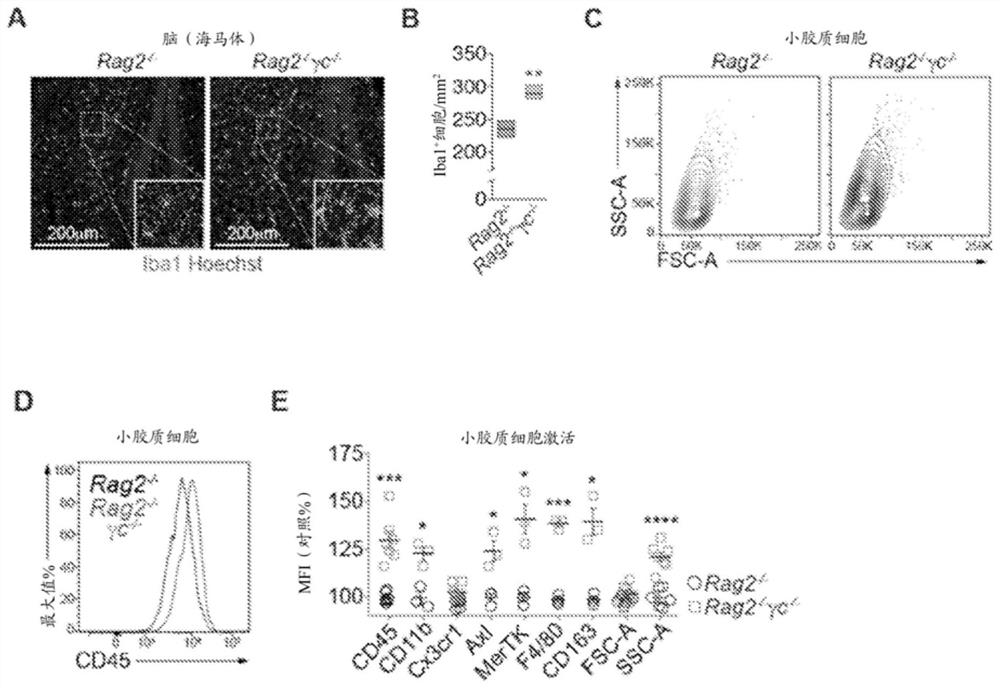
[0154] Embodiment 1 is a method of reducing or inhibiting microglial activation in a subject in need thereof, the method comprising administering to the subject a therapeutically effective amount of activated type II innate lymphoid cells (ILC2 ).
[0155] Embodiment 1a is a method of reducing the permeability of the blood-brain barrier (BBB) in a subject in need thereof, the method comprising administering to the subject a therapeutically effective amount of activated type II innate lymphoid cells (ILC2).
[0156] Embodiment 2 is the method according to embodiment 1 or 1a, wherein the ILC2 is obtained by combining the ILC2 with at least one selected from IL-33, IL-25, IL-2, IL-7 or combinations thereof Cytokine exposure to activate.
[0157] Embodiment 2a is the method according to embodiment 2, wherein said ILC2 is activated by contacting said ILC2 with two cytokines selected from the group con...
Embodiment approach 21c
[0228] Embodiment 21c is a method of identifying an agent useful for reducing BBB permeability in a subject in need thereof, the method comprising:
[0229] (1) contacting innate lymphoid cells (ILC2) with the agent; and
[0230] (2) measuring the level of Timp1 produced by said ILC2;
[0231] wherein an increase in the amount of Timpl produced by the ILC2 compared to a control level indicates that the agent is useful for reducing BBB permeability in a subject in need thereof.
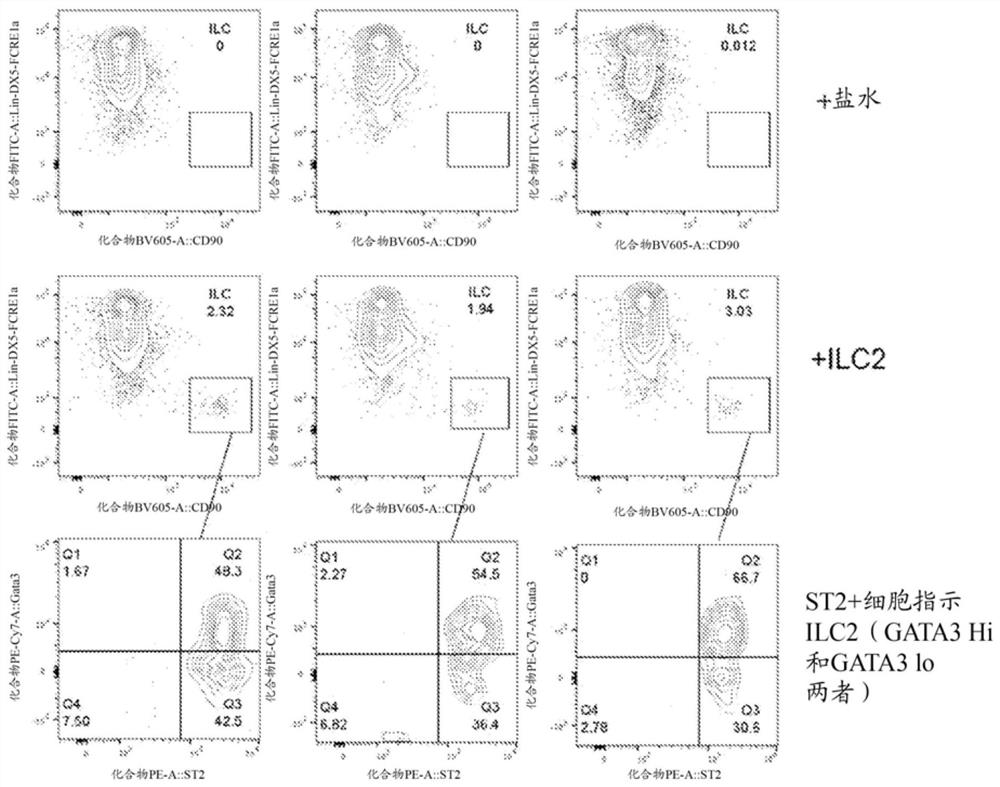
[0232] Embodiment 22 is the method according to any one of embodiments 1 to 13, the pharmaceutical composition according to any one of embodiments 14 to 16, or the method according to any one of embodiments 17 to 21c The method, wherein the ILC2 expresses one or more of CD90, ICOS, IL7Ra (CD127), CD161, ST2, stem cell antigen 1 (Sca1), IL2Ra (CD25) and CRTH2 and is negative for the expression of Lin.
[0233] Embodiment 22a is the method of embodiment 22, wherein the ILC2 expresses two of CD90, ICOS,...
Embodiment 1
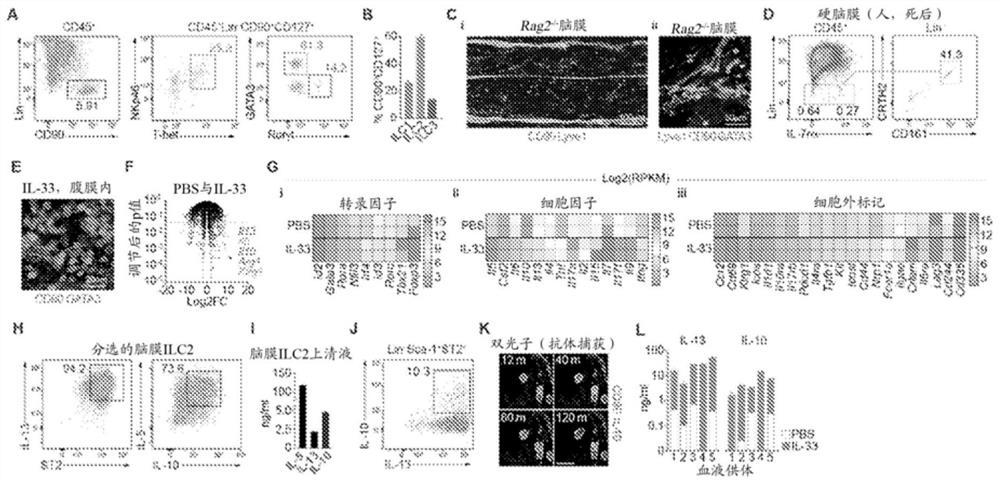
[0249] Example 1. Enrichment of ILC2 in meninges
[0250] Transcription factor markers of CD45+Lin–FCεr1a–DX5–CD90+IL7rα+ cells ( figure 1 A) Used to resolve ILC1 (Tbet+), ILC2 (Gata3Hi) and ILC3 (RORγt+Gata3– / lo) subpopulations. First, meningeal innate lymphoid cells (mILCs) were analyzed to determine the most abundant type. Naïve mouse brains were carefully dissected and immediately fixed in 4% paraformaldehyde. After fixation at 4°C for 48 hours (hr), the brain was incubated in 30% sucrose in H 2 O in solution for 24 hr and then stored at -80 °C. The calvaria was placed in 20 ml of fluorescence activated cell sorting (FACS) buffer (DMEM / F-12 + 2% FBS) in a petri dish and the meninges were carefully peeled off using forceps. Place the meninges in a 70 μm cell strainer and gently dissociate using the reverse side of the plunger of a syringe with (minimum 100) strokes. Cells were then washed three times through the filter to further release cells from the dissociated t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com