A kind of preparation method of amantadine
A technology of amantadine and amantadine, which is applied in the field of amantadine preparation, can solve problems such as low yield, high quality requirements, and difficulty in meeting requirements, and achieve the goals of reducing the discharge of three wastes, reducing production costs, and increasing reaction yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Synthesis of polyionic liquid catalyst PIL-1
[0044] Add 2-vinylpyridine (21.0g, 0.2mol) (or 4-vinylpyridine (21.0g, 0.2mol)) to 40ml of tetrahydrofuran, and then add 1,3-propanesultone (24.4g, 0.2mol) ), the mixture was stirred at room temperature (usually 25 °C) for 72 h, forming a white solid. It was filtered, washed repeatedly with diethyl ether (30 ml×3), and then the solid was vacuum dried (100° C., 1.0 Pa) to obtain a pure white solid (90% yield). An equal amount of sulfuric acid was added to the obtained solid, and the mixture was stirred at 60 °C for 4 h to form an ionic liquid monomer.
[0045] The reaction equation is as follows:
[0046]
[0047] The ionic liquid monomer (6.28g, 20mmol), 40mL ethanol, 4mLH 2 O and 0.02 g of azobisisobutyronitrile were mixed to form a solution, and after stirring at 70 °C for 4 h, 1,3-divinylbenzene (2.60 g, 20 mmol) and azobisisobutyronitrile (0.02 g) were added to it The reaction solution was stirred for ...
Embodiment 2
[0054] Example 2: Synthesis of 1-acetamidoadamantane (compound 2)
[0055] The polyionic liquid PIL-1 (1 g) was added to a mixture of acetonitrile (7.533 g, 0.1835 mol) and adamantane (10 g, 0.0734 mol) in 1,2-dichloroethane (32 ml), and then 98% was slowly added dropwise of sulfuric acid (7.193 g, 0.0734 mol), and the reaction was stirred at 60 °C for a total time of 19 h. After the reaction, the acid catalyst was removed by suction filtration, then distilled under reduced pressure at 10°C, vacuum-0.09MPa, distilled to a temperature of 60°C and vacuum-0.1Mpa in the bottle, no fraction was distilled in the receiving bottle, and then put into a water bath to cool down, and the bottle was cooled. 200 g of deionized water was added dropwise at an inner temperature of 10°C, and the dropwise addition was completed after 1 hour, and the inner temperature of the bottle was maintained at 10°C and stirring was continued for 1 hour. Turn off stirring, suction filtration, and rinse the ...
Embodiment 3
[0074] Example 3: Synthesis of Amantadine (Compound 3)
[0075] The product prepared above, 1-acetamidoadamantane (13.329 g, 0.0689 mol), was added to a mixture of ethylene glycol (20 ml) and granular sodium hydroxide (16.536 g, 0.4134 mol), which was heated at 135°C for 12 After the reaction was completed, 50 ml of deionized water was added to quench, and the mixture was stirred for 1 h. with CH 2 Cl 2 (20ml×5) extraction, the organic layer was yellow, and then washed with deionized water for 20ml×3. The product was a yellow-brown solid 9.379 g. GC purity 99%. Yield 90%. The theoretical yield is 10.421 g.
[0076] The reaction equations involved are as follows:
[0077]
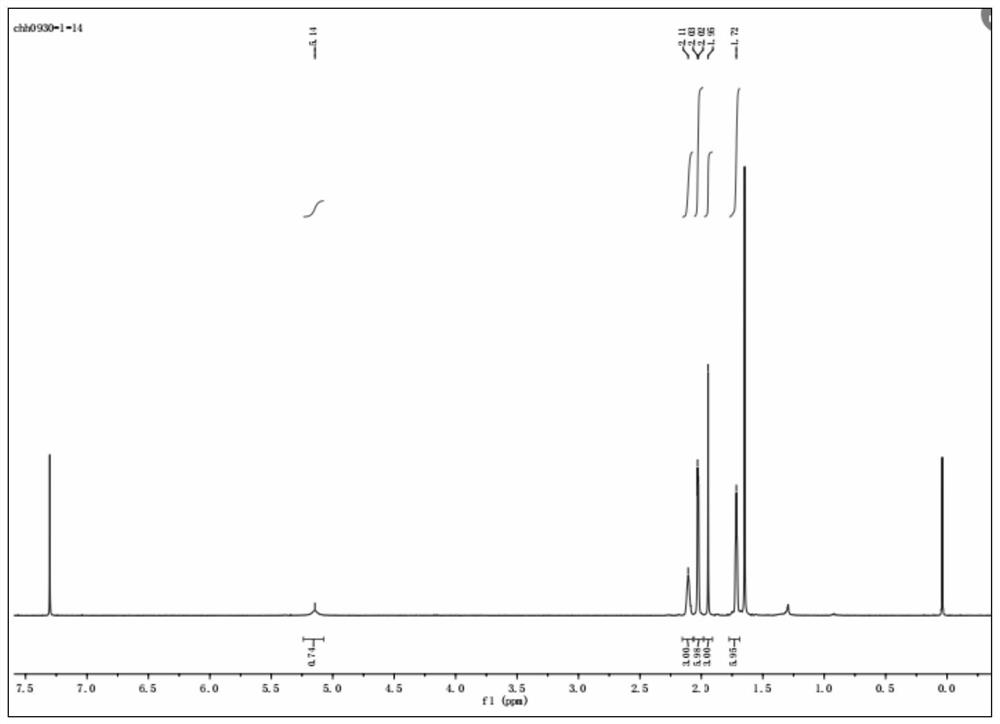
[0078] Product structure confirmation:
[0079] The mass spectrum of the amantadine prepared in Example 3 is as follows Figure 4 and as shown in the table below:
[0080] Peak List
[0081] m / z z Abund 57.1 3460.08 58.1 1 1234.92 77.1 2261.54 79.1 2 1168.7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bronsted acidity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap