Methods and compositions for inhibition of egf/egfr pathway in cobination with anaplastic lymphoma kinase inhibitors
A technology of inhibitors and protocols, applied in drug combinations, chemical instruments and methods, anti-animal/human immunoglobulins, etc., can solve problems such as patient relapse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0193] Example 1: Effects of combined use of ALK inhibitors and anti-EGF antibodies in non-small cell lung cancer cell lines.
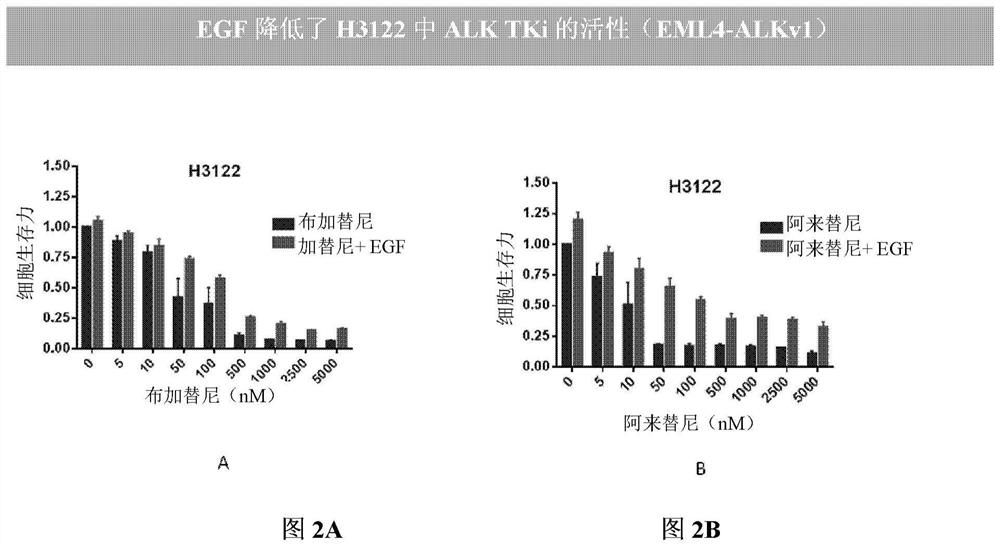
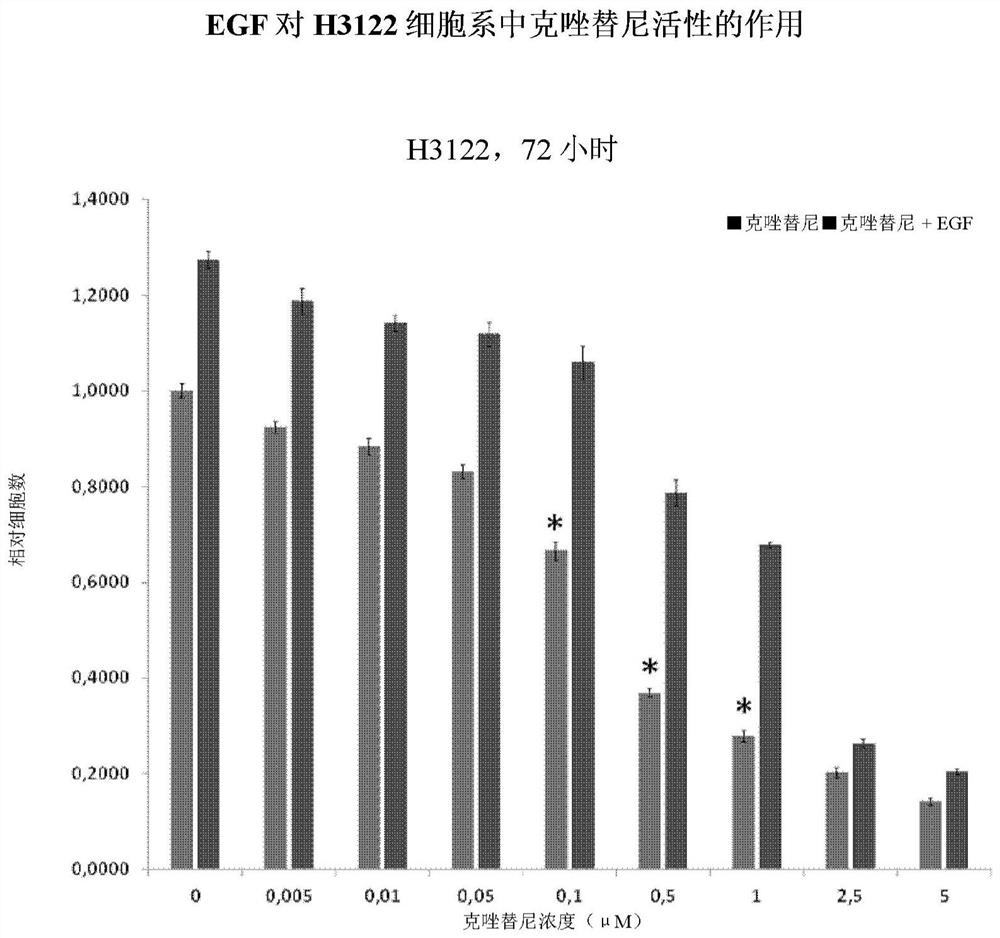
[0194] Compared with EGFR-mutant patients treated with EGFR-TKI, patients treated with ALK-TKI achieved longer progression-free survival. Clinically, 20-30% of patients develop resistance rapidly, but it is unknown whether these patients develop resistance or never respond. The role of circulating EGF in these patients is unclear. The present invention proposes the effect of EGF on TKI resistance using two cell lines containing major ALK rearrangements in NSCLC patients.
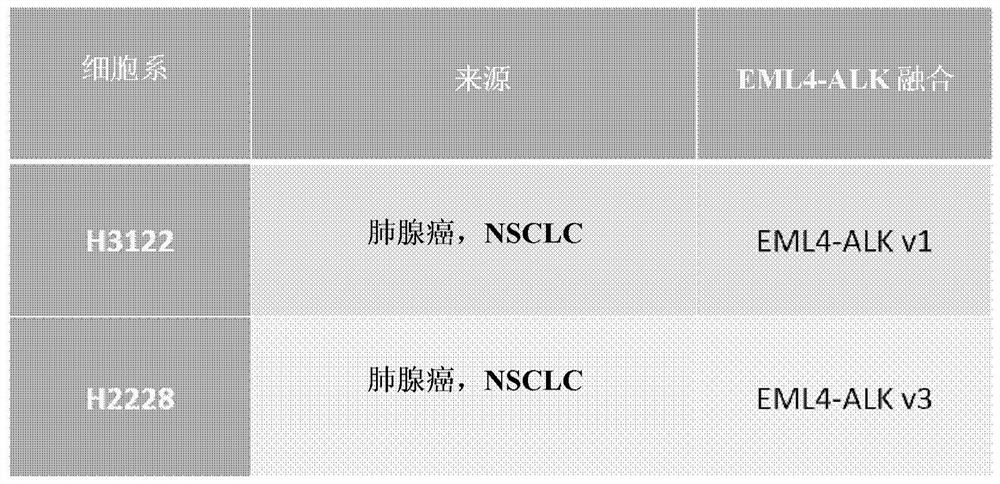
[0195] The combination of the BVN22E antibody with the following ALK-TKIs—crizotinib, alectinib, and Brigantinib—was tested on two cell lines harboring major ALK rearrangements in NSCLC patients. An ALK "rearrangement" refers to a series of different mutations. These cell lines such as figure 1 Shown: H3122 lung adenocarcinoma, which contains EML4-ALK fusion variant v1, and H222...
Embodiment 2
[0209] Example 2: Evaluation of the combined use of anti-EGF antibodies and trametinib in BRAF and KRAS mutant cell lines.
[0210] Colorectal cancer (CRC) is not only one of the most common neoplastic cancers, but also presents particular challenges in terms of treatment. Clinically, CRC patients without KRAS mutations can receive EGF-targeted therapies such as cetuximab and erlotinib. However, a large proportion of CRC patients have KRAS mutations, BRAF mutations, or PIK3CA mutations. There are currently no effective treatments for these patients. Chemotherapy and angiogenesis-targeted therapy are commonly used treatments, but they also have significant drawbacks.
[0211] To begin to address the therapeutic needs of CRC patients with KRAS, BRAF, or PIK3CA mutations, in vitro assays of EGF antibodies in combination with the MEK inhibitor trametinib were performed in cell lines with the aforementioned mutations. All experiments were performed in combination with BVN22E ant...
Embodiment 3
[0231] Example 3: Serum evaluation of patients vaccinated with EGF cancer vaccine in SW900 cells.
[0232] The effect of anti-EGF serum from a human patient (22180004) on pEGFR in SW900 wild-type cells in the presence of EGF was observed. 2a = patient before vaccination, 3d = patient after vaccination. In the presence of anti-EGF serum, complete inhibition of pEGFR activation was observed, along with some reduction in pERK1 / 2, see Figure 70 . The effect of human patient anti-EGF serum on pEGFR was observed in SW900 wild-type cells in the presence of EGF in two additional patients, see Figure 71 . Significant inhibition of pEGFR and pERK1 / 2 activation was observed in patient 27030004, while lesser inhibition of pEGFR and pERK1 / 2 was observed in patient 29080005. The anti-EGF serum results of the other three patients were as follows: Figure 72 shown. Significant inhibition of pEGFR and pERK1 / 2 signaling was observed for two of these patients (29040007 and 3308020). For...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com