Star-shaped poly(propylene fumarate) copolymers for 3D printing applications
A technology of propylene glycol fumarate and star-shaped copolymer, which can be used in prosthesis, additive processing, medical science, etc., and can solve problems such as low viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0137] The following examples are provided to more fully illustrate the invention but should not be construed as limiting its scope. Furthermore, while some of the examples may include conclusions about the manner in which the invention may function, the inventors do not intend to be bound by these conclusions, but set them out only as possible explanations. Also, unless indicated by use of the past tense, the representation of an example does not imply that an experiment or procedure has or has not been performed or that a result has or was not actually obtained. Efforts have been made to ensure accuracy with respect to numbers used (eg, amounts, temperature) but some experimental errors and deviations may be present. Unless indicated otherwise, parts are parts by weight, molecular weight is number average molecular weight, temperature is in degrees Centigrade, viscosity is in Pa.s, and pressure is at or near atmospheric.
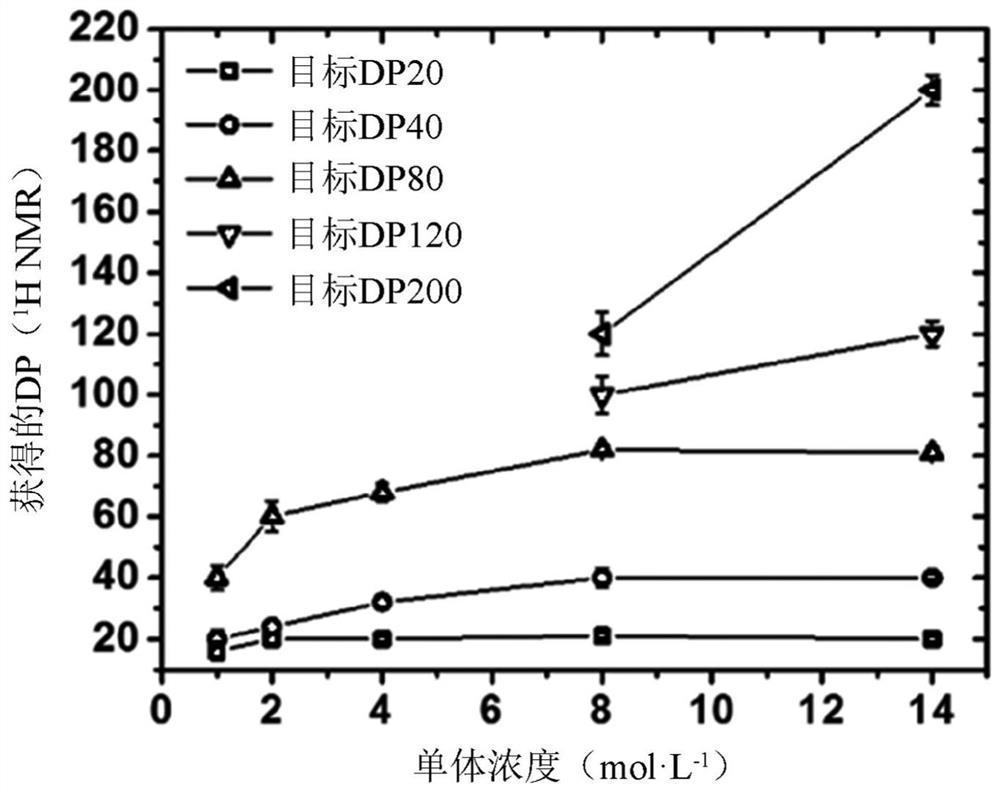
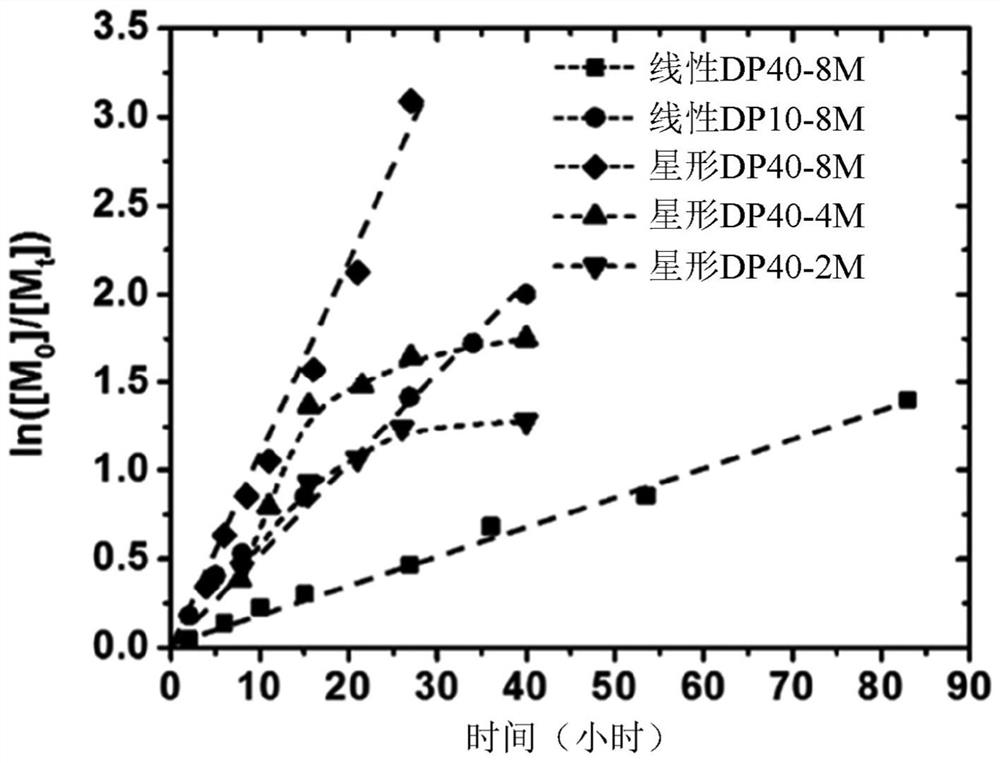
[0138] In the first set of experiments (Examples 1-...
example 1
[0146]Synthesis of 4-armed star poly(trimethylene maleate) of total DP20 with a meso-erythritol core
[0147] Meso-erythritol (112.12g.mol -1 , 249.1mg, 2.04mmol), Mg(BHT) 2 (THF) 2 (604.95g.mol -1 , 246.8mg, 4.08×10 -1 mmol), anhydrous toluene (10.2mL), MAn (98.06g.mol -1 , 4.0g, 40.8mmol) and PO (58.08g.mol -1 , 2.85 mL, 40.8 mmol) were introduced into a flame-dried Schlenk bottle in this order. The Schlenk bottle was sealed with a PTFE stopper and removed from the glove box. The solution was stirred at 80°C for 24 hours. The resulting copolymer was recovered by precipitation in diethyl ether and dried under vacuum to give a sticky beige powder. Yield: 88%.
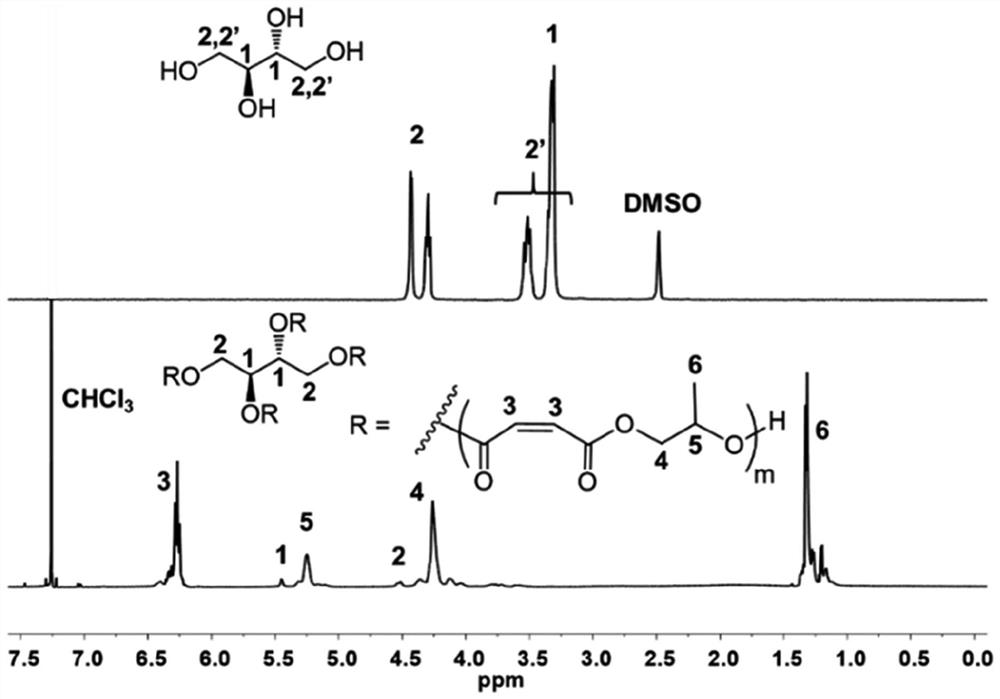
[0148] pass 1 H NMR (500MHz, 298K, CDCl 3 ) to characterize the resulting 4-arm star poly(trimethylene maleate) copolymer: 6.42-6.18 (m, 42.1H, C=OCHCHC=O), 5.44 (m, 2H, CH(OR)CH 2 OR), 5.36-5.17 (m, 21.2H, CH 2 CHCH 3 O), 4.55-4.47(d, 1.7H, CH(OR)CHHOR), 4.45-4.00(m, 44.9H, OCH 2 CHCH 3 ), 1.41-1.23(m, ...
example 2
[0150] Synthesis of 4-armed star poly(trimethylene maleate) of total DP40 with a meso-erythritol core
[0151] Meso-erythritol (112.12g.mol -1 , 124.5mg, 1.02mmol), Mg(BHT) 2 (THF) 2 (604.95g.mol -1 , 123.4mg, 2.04×10 -1 mmol), anhydrous toluene (10.2mL), MAn (98.06g.mol -1 , 4.0g, 40.8mmol) and PO (58.08g.mol -1 , 2.85 mL, 40.8 mmol) were introduced into a flame-dried Schlenk bottle in this order. The Schlenk bottle was sealed with a PTFE stopper and removed from the glove box. The solution was stirred at 80°C for 48 hours. The resulting copolymer was recovered by precipitation in diethyl ether and dried under vacuum to give a sticky beige powder. Yield: 78%
[0152] pass 1 H NMR (500MHz, 298K, CDCl 3 ) to characterize the resulting 4-arm star poly(trimethylene maleate) copolymer: 6.42-6.18 (m, 79.3H, C=OCHCHC=O), 5.44 (m, 2H, CH(OR)CH 2 OR), 5.36-5.17(m, 37.5H, CH 2 CHCH 3 O), 4.55-4.47(d, 2.1H, CH(OR)CHHOR), 4.45-4.00(m, 86.5H, OCH 2 CHCH 3 ), 1.41-1.25(m, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com