A class of active peptides derived from caprin1 and their applications
An active peptide and active technology, applied in the fields of application, animal/human peptides, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
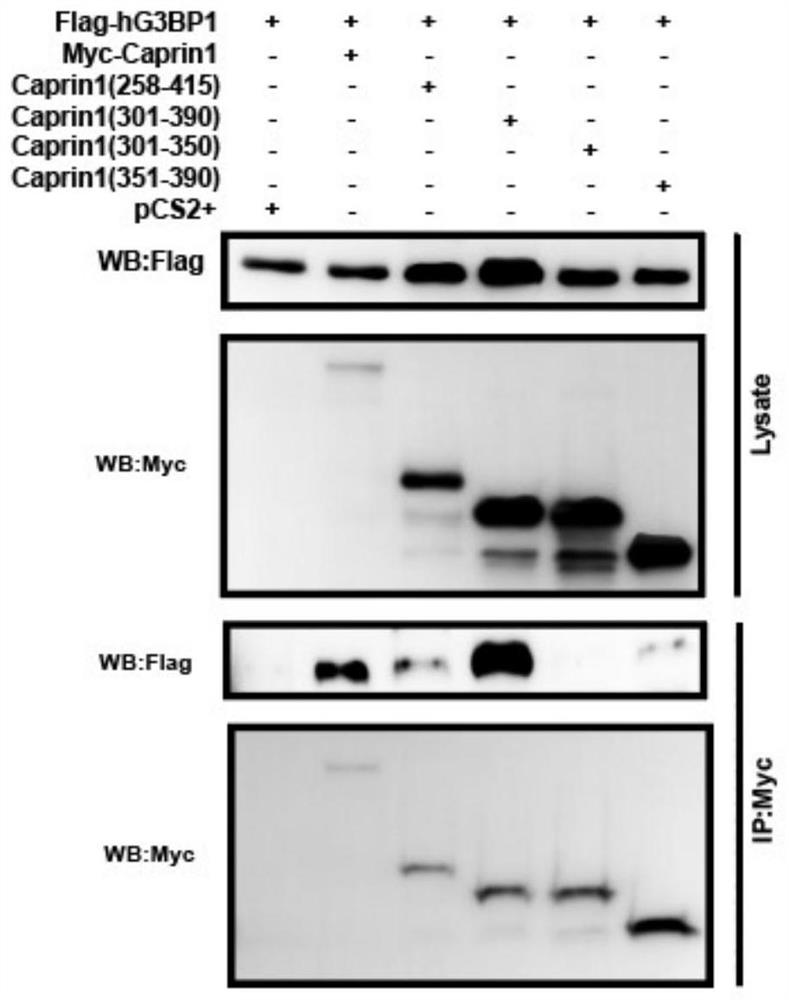
[0042] Example 1 Design of polypeptide SGs inhibitor based on blocking the interaction between Caprin1 and G3BP1 1. According to research reports, the interaction between Caprin1 and G3BP1, two nucleating proteins of SGs, can promote the assembly of SGs. The present invention explores the minimal G3BP1 binding region of Caprin1 protein, and designs four polypeptide fragments according to Caprin1 protein: Caprin1 (258-415), Caprin1 (301-390), Caprin1 (301-350), Caprin1 (351-390). Shanghai Jill Biochemical Company was entrusted to synthesize the above-mentioned polypeptide fragments.
[0043] 2. Construct Caprin1 and hG3BP1 expression plasmids, design and synthesize the following primers:
[0044] G3BP1:
[0045] G3BP1-F 5'-atggtgatggagaagcctagtcccctgct-3' (SEQ ID No: 14);
[0046] G3BP1-Xba-R 5'-ccatctagattcactgccgtggcgcaagcc-3' (SEQ ID No: 15);
[0047] Caprin1:
[0048] Caprin1-F 5'-atgccctcggccaccagccaca-3' (SEQ ID No: 16);
[0049] Caprin1-Xba-R 5'-tcttctagaaacatattatg...
Embodiment 2
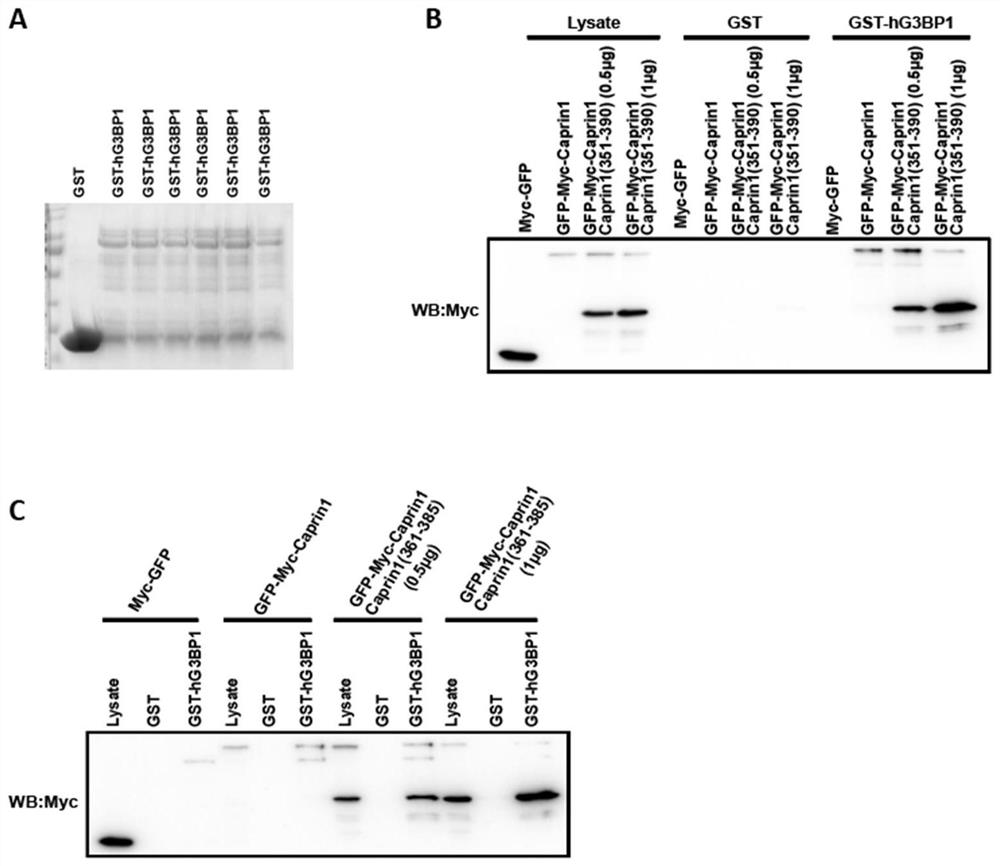
[0057] Example 2 Investigating the ability of the active peptide derived from Caprin1 of the present invention to compete with the protein Caprin1 for binding to G3BP1
[0058] Based on the research results in Example 1, Caprin1 (361-385) with a shorter sequence was designed on the basis of Caprin1 (351-390).
[0059] 1. Construction of Caprin1 (361-385) and Caprin1 (351-390) expression plasmids
[0060] Design and synthesize the following primers:
[0061] Caprin1 (351-390):
[0062] Caprin1-351F 5'-gatccccttgtgagaagacagcgagtacaa-3' (SEQ ID No: 18);
[0063] Caprin1-Xba-390R 5'-ctgtctagatacaatggcaggatcaagtgt-3' (SEQ ID No: 19);
[0064] Caprin1 (361-385):
[0065] Caprin1-361F 5'-gaccttatggcacaaatgcagggtccctat-3' (SEQ ID No: 20);
[0066] Caprin1-Xba-385R 5'-tgatctagaaagtgtctgattttcaaaatc-3' (SEQ ID No: 21).
[0067] With reference to "Molecular Cloning Experiment Guide (Fourth Edition)" (Cold Spring Harbor Laboratory Press / Science Press), the pCS2-Myc-hCaprin...
Embodiment 3
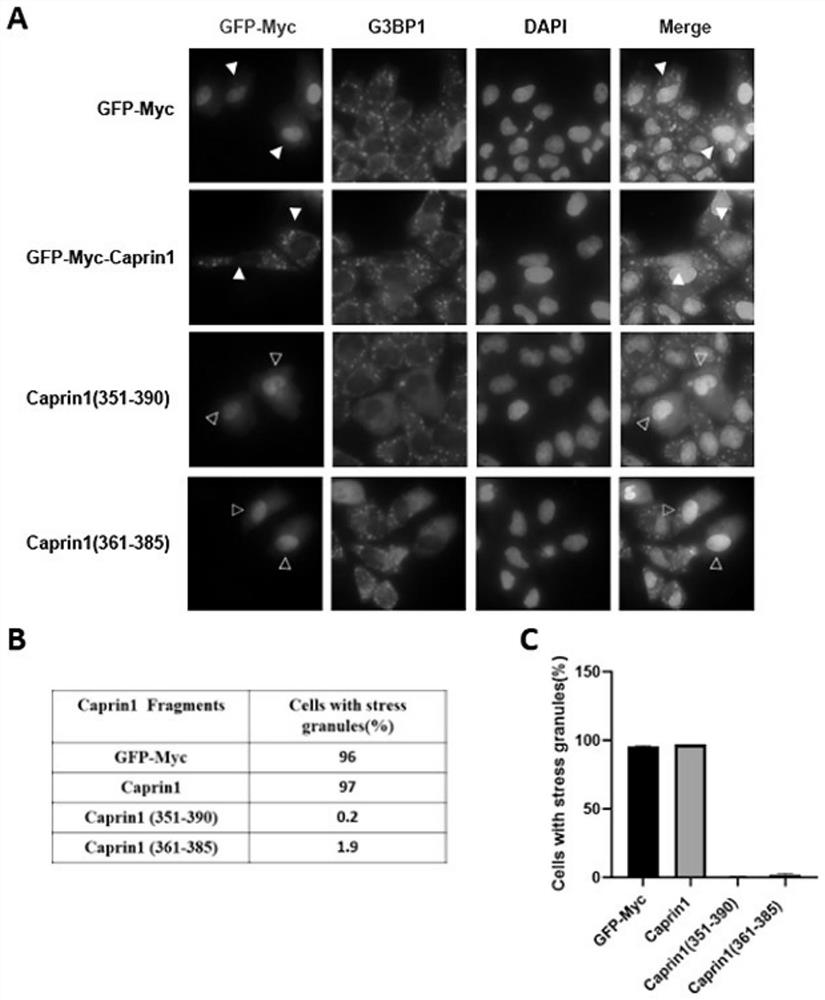
[0082] Example 3 Investigating the activity of Caprin1 (361-385) and Caprin1 (351-390) in inhibiting SGs HeLa cell lines were seeded in 6-well plates, and when the cell confluence reached about 60%, the expression Caprin1 constructed in Example 2 was (361-385) and Caprin1 (351-390) plasmids were transfected into HeLa cells and overexpressed in the cells. When the cell confluence reaches about 80%, take 12 mL of cell culture medium into a 15 mL centrifuge tube, add 12 μL of 0.5 mM AS, mix well, replace the medium in the 6-well plate, and stimulate HeLa cells to form SGs (using endogenous G3BP1 as the SGs). Mark). Put into a carbon dioxide cell incubator and continue to culture for 45min. Remove the 6-well plate for immunofluorescence experiments.
[0083] The result is as image 3 As shown, the results showed that overexpression of Caprin1(351-390), Caprin1(361-385) inhibited AS-induced SGs ( image 3 A). In order to ensure the authenticity and reproducibility of the exper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com