An expression cassette for recombinant expression of echinocandin b deacylase and its application
A technology of deacylase and echinocandin, applied in the field of bioengineering, can solve the problems of inactive protein, Escherichia coli growth inhibition, unsatisfactory activity and solubility, etc., and achieve the effect of high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
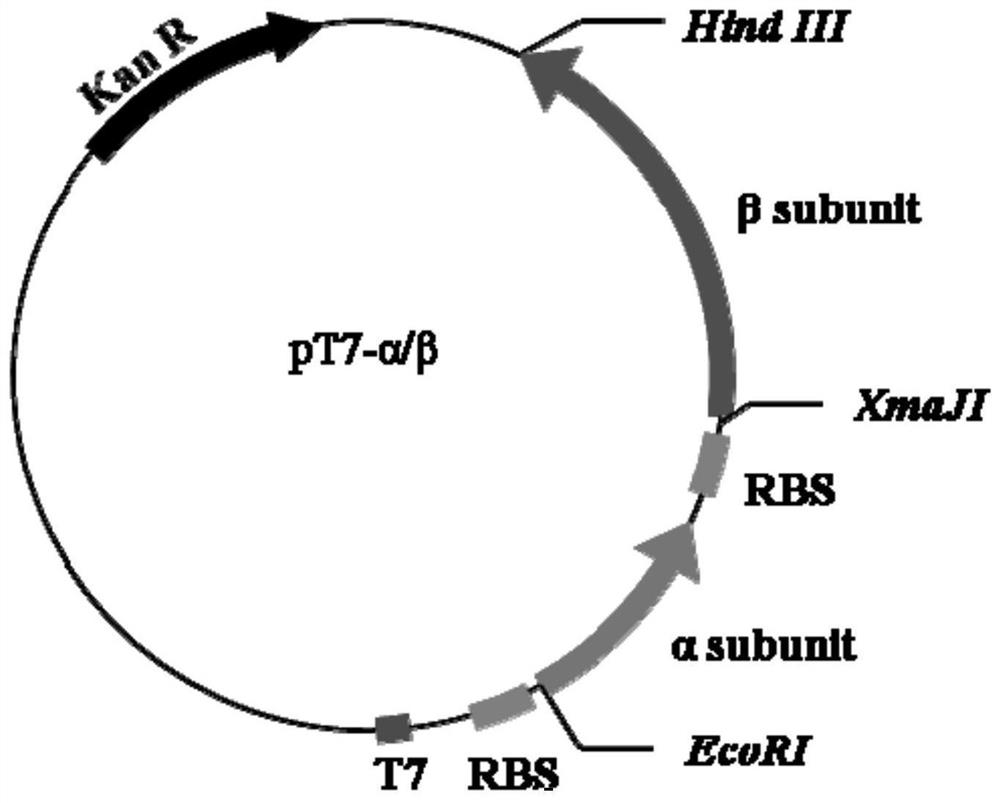
[0030] Construction of recombinant strain E.coli BL21(DE3) / pT7-α / β.
[0031] 1. Codon optimization and synthesis of the gene encoding echinocandin B deacylase
[0032] The echinocandin B deacylase was obtained from a plasmid preserved in the laboratory.
[0033] According to the codon preference of Escherichia coli, the codon-optimized echinocandin B deacylase coding nucleotide (DNA) sequence was artificially designed and synthesized, and the target gene fragment was obtained after sequencing verification.
[0034] The amino acid sequence of echinocandin B deacylase is shown in SEQ ID NO.1, and its optimized nucleic acid sequence is shown in SEQ ID NO.2, wherein the signal peptide coding sequence is 1-96bp, and the alpha subunit coding sequence is 97 -645bp (optimized sequence, the nucleotide sequence after adding TAA as the stop codon is shown in SEQ ID No.6), the connecting peptide coding sequence is 646-690bp, and the β subunit coding sequence is 691-2364bp (after optimizi...
Embodiment 2
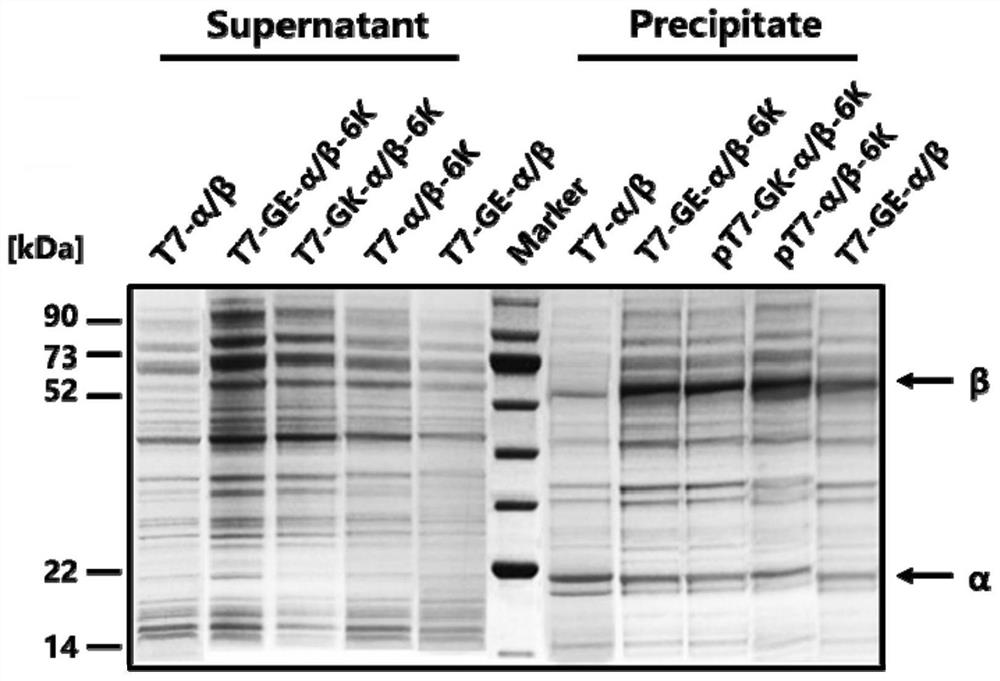
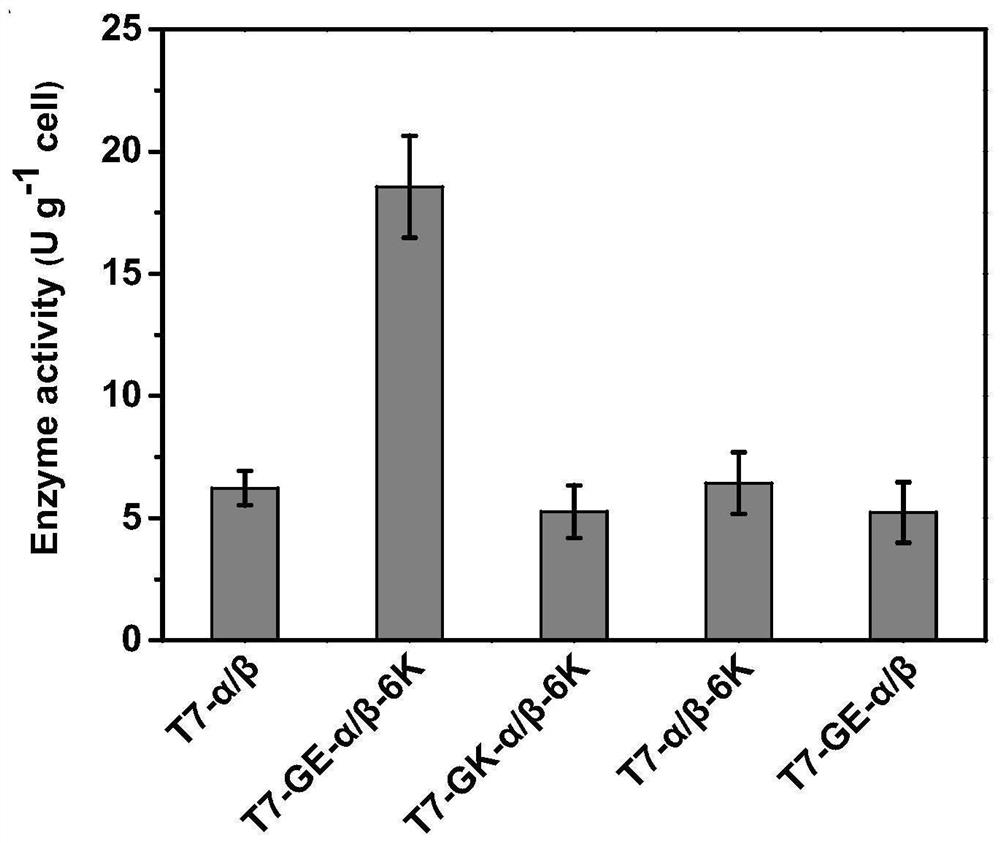
[0055] Construction of recombinant strains T7-α / β-6K, T7-GE-α / β-6K, T7-GK-α / β-6K, pT7-GE-α / β.
[0056] 1. Construction of recombinant strains containing different lytic short peptides
[0057] Wherein the nucleic acid sequence of the short peptide is:
[0058] 6K solubilizing short peptide (amino acid sequence KKKKKK): aaaaaaaaaaaaaaaaaaa;
[0059] GE solubilizing short peptide (amino acid sequence GEGEG): ggagaaggagaagga;
[0060] GK solubilizing short peptide (amino acid sequence GKGKG): ggtaaaggtaaaggt.
[0061] The primer sequences used are:
[0062] GKGKG Fusion-F:
[0063] AGCGCAGGCCggtaaaggtaaaggtCATGATGGTGGCTACGCGGCGCTGAT;
[0064] GKGKG Fusion-R:
[0065] GCGCCGCGTAGCCACCATCATGacctttaccttaccGGCCTGCGCTACGGTAGCGAAA;
[0066] GEGEG Fusion-F:
[0067] TAGCGCAGGCCggagaaggagaaggaCATGATGGTGGCTACGCGGCGCTGATCCG;
[0068] GEGEG Fusion-R:
[0069] GCGCCGCGTAGCCACCATCATGtccttctccttctccGGCCTGCGCTACGGTAGCGAA;
[0070] 6K-F:
[0071] GAAGCGCCGGACGCGAAAAAAAAAAAAAAAAAATAAg...
Embodiment 3
[0080] Optimization of fermentation conditions of recombinant strain T7-GE-α / β-6K.
[0081] 1. Optimization of the inducer concentration of the recombinant strain T7-GE-α / β-6K.
[0082] It is well known that, in addition to the expression host itself, expression conditions largely affect the productivity of recombinant enzymes. Therefore, we investigated the effects of IPTG concentration, induction temperature and induction time on the activity of ECBD-expressing crude enzyme solutions. Enzyme activity increased with increasing IPTG concentration from 0 mM to 0.4 mM, but further increases in IPTG concentration decreased the activity. When the concentration of IPTG exceeds 0.6 mM, the soluble forms of α and β subunits are significantly reduced. The viability of the cells correlates with the expression level of the soluble form of the alpha subunit. The strongest growth inhibition occurred at 0.2 mM IPTG. The highest enzyme activity was obtained at 0.4mM IPTG ( Figure 5 ),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com