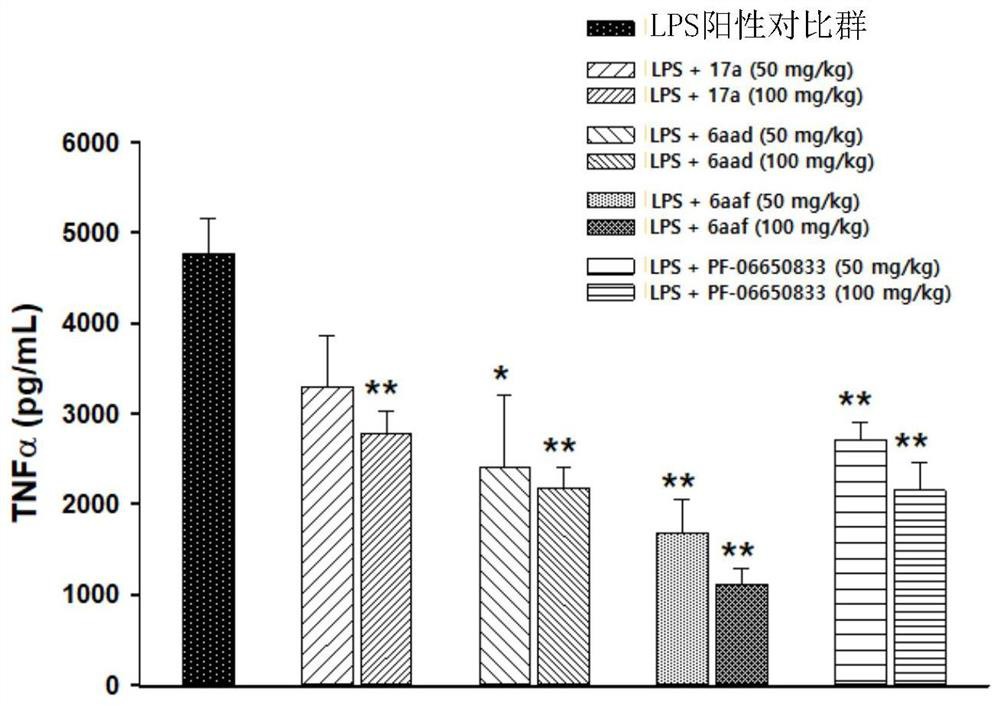
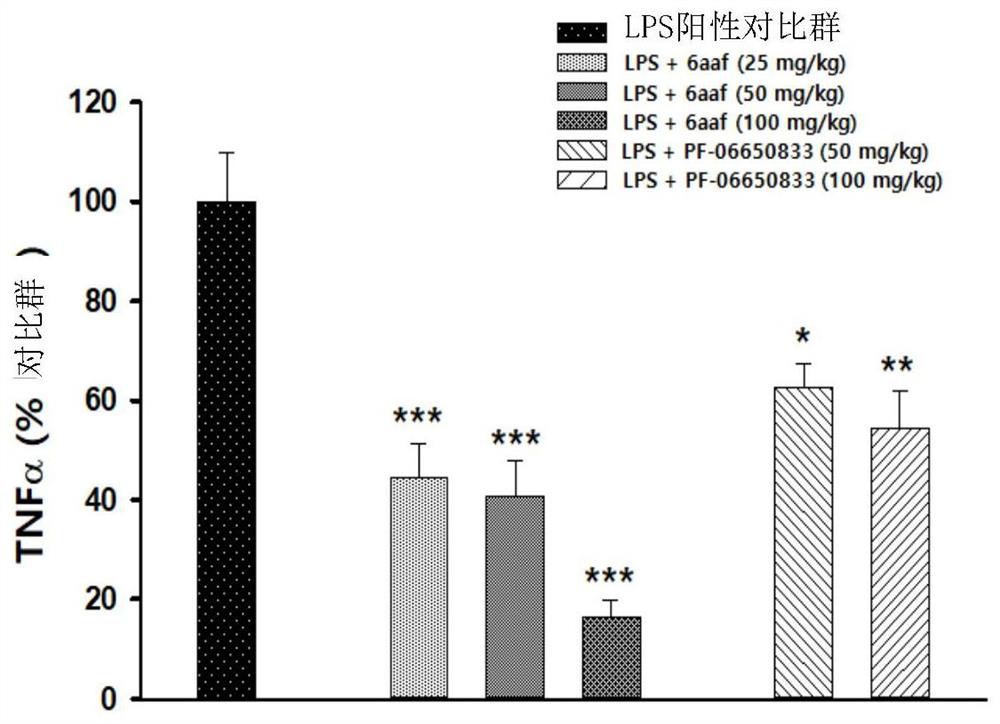
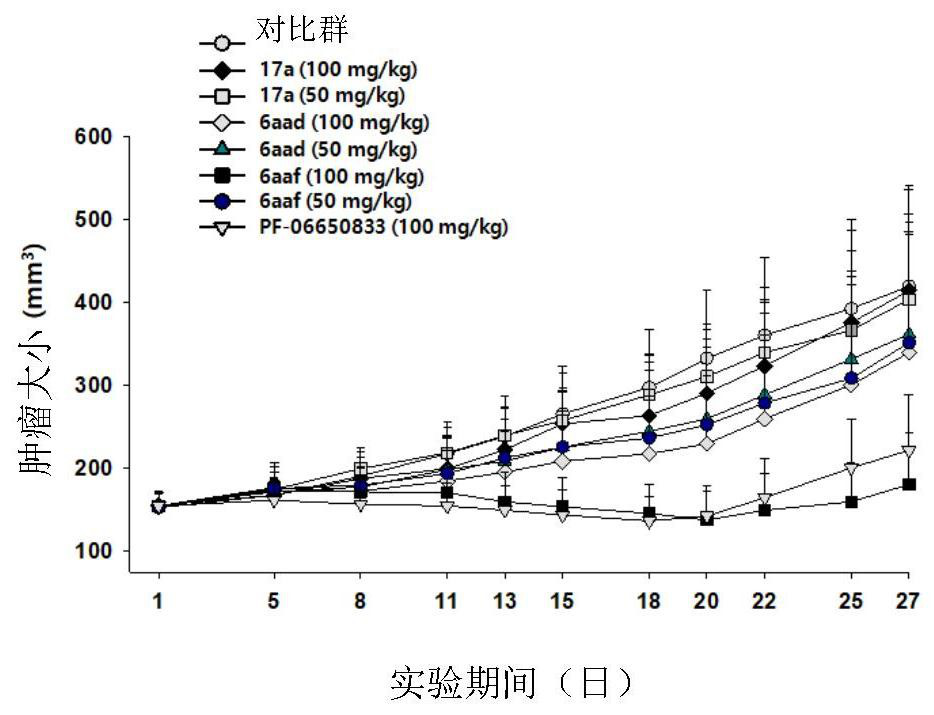
Novel tricyclic compound as irak4 inhibitor
A cyclic compound, chemical formula technology, applied in the field of new tricyclic compounds as IRAK4 inhibitors, can solve the problems of inability to greatly improve the disease, high cost, and achieve excellent inhibitory activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 16
[0132] [Production Example 1] Production of 6-bromo-4-chloro-9H-pyrimido[4,5-b]indole (compound 4a)
[0133]
[0134] Step 1. Production of compound 2a
[0135] After compound 1a (36 g, 143 mmol) was dissolved in dichloromethane (300 ml), triethylamine (40 mL, 287 mmol) was added, and trifluoroacetic anhydride (30 mL, 216 mmol) was added slowly at 0°C. Wash with distilled water (2×300mL) after the reaction finishes, then wash with sodium sulfate (Na 2 SO 4 ) to remove moisture and concentrate under reduced pressure to obtain 50 g of trifluoroacetamide (trifluoroacetamide) derivatives.
[0136] Dissolve the trifluoroacetamide derivative in DMSO (dimethyl sulfoxide)-distilled water (1:1; 280 mL), and add methyl cyanoacetate (15 mL, 170 mmol), DL-proline (3.3 g, 18.8 mmol ), K 2 CO 3 (40g, 289mmol), CuI (2.73g, 14.3mmol), and stirred at 60°C for 14 hours. After cooling the reactant to room temperature, the solid was filtered, washed with methanol (2×100 mL), and the filt...
manufacture example 2
[0144] [Production Example 2] Production of ethyl 4-chloro-9H-pyrimido[4,5-b]indole-6-carboxylic acid methyl ester (compound 4b)
[0145]
[0146] Compound 4b was produced from compound 1b in three steps in the same manner as in Production Example 1 above.
[0147] 4b: 1 H-NMR (300MHz, DMSO-d 6 )δ1.38(t, J=7.1Hz, 3H), 4.38(q, J=7.1Hz, 2H), 7.70(d, J=8.6Hz, 1H), 8.19(dd, J=8.6, 1.3Hz, 1H), 8.80(s,1H), 8.85(s,1H), 13.13(s,1H).
manufacture example 37
[0148] [Production Example 3] Production of 7-bromo-4-chloro-9H-pyrimido[4,5-b]indole (Compound 4c)
[0149] Compound 4c was produced from compound 1c in three steps in the same manner as in Production Example 1 above.
[0150] 4c: 1 H-NMR (300MHz, DMSO-d 6 )δ7.60(dd, J=8.4,1.8Hz,1H),7.81(d,J=1.7Hz,1H),8.22(d,J=8.5Hz,1H),8.83(s,1H),12.94( s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com