Synthetic method of loratinib intermediate
A synthetic method, the technology of lorlatinib, applied in the field of medicine, can solve the problems of unfavorable industrial production, unavailable raw materials, high cost, etc., and achieve the effect of short steps, low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
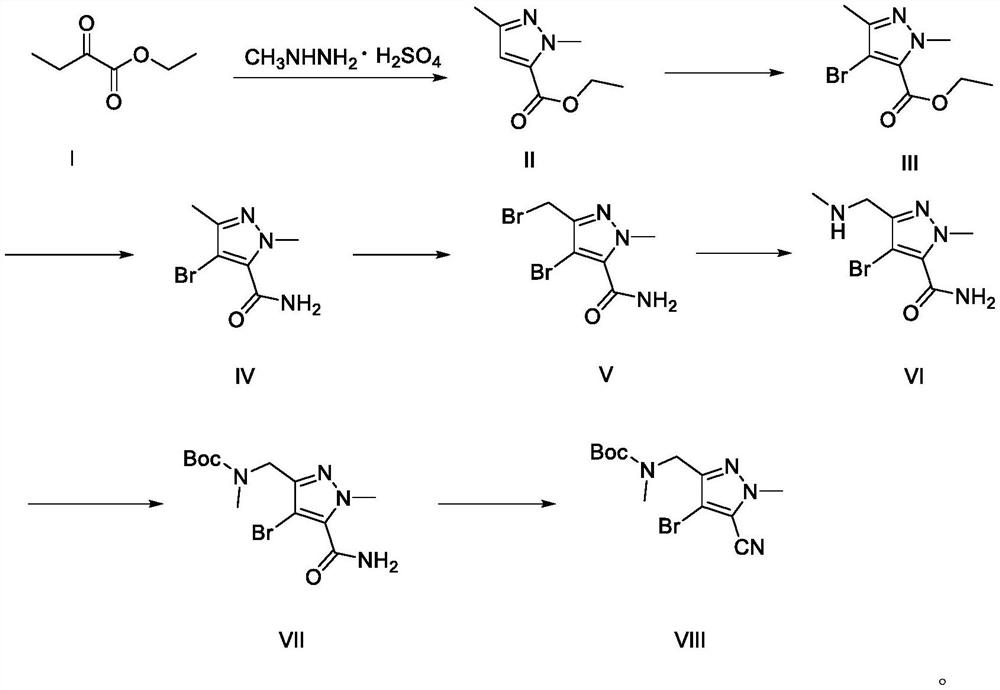
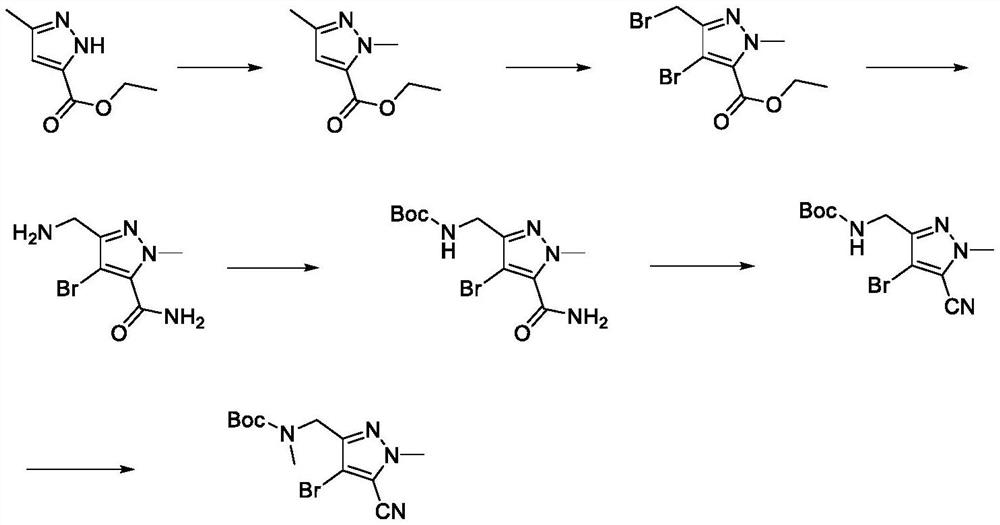
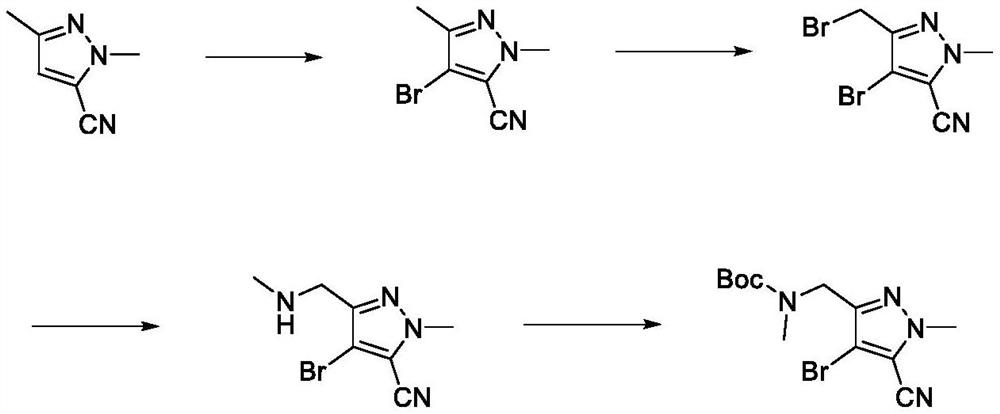
[0049] A synthetic method of lorlatinib intermediate, comprising the following steps:
[0050] (1) Preparation of ethyl 1,3-dimethyl-1H-pyrazole-5-carboxylate (Ⅱ)
[0051]
[0052] Add 50ml of absolute ethanol to a 250ml three-neck flask, add 12.2g (84.5mmol) of methylhydrazine sulfate, cool down to 0-5°C, and dropwise add a mixed solution of 16.7g (245.9mmol) of sodium ethoxide and 50ml of absolute ethanol After dropping, keep the temperature at 0-5°C, add 10.0 g (76.8 mmol) of acetone ethyl oxalate dropwise, and react at 0-5°C for 4 hours after dropping. Post-reaction treatment, add 100ml of water, evaporate ethanol under reduced pressure, add dichloromethane for extraction, dry and concentrate to obtain 13g of light yellow liquid, distill under reduced pressure to obtain 11.5g of colorless liquid, yield 89.0%, the purity of the product by HPLC 99.0%.
[0053] (2) Preparation of ethyl 4-bromo-1,3-dimethyl-1H-pyrazole-5-carboxylate (Ⅲ)
[0054]
[0055] Add 100ml of ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap