Diosgenin hydroxamic acid derivatives and their preparation methods and applications
A technology of aglycone hydroxime and derivatives is applied in the field of diosgenin hydroxamic acid derivatives and their preparation, and can solve the problems of low bioavailability, high cytotoxicity, and narrow application range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
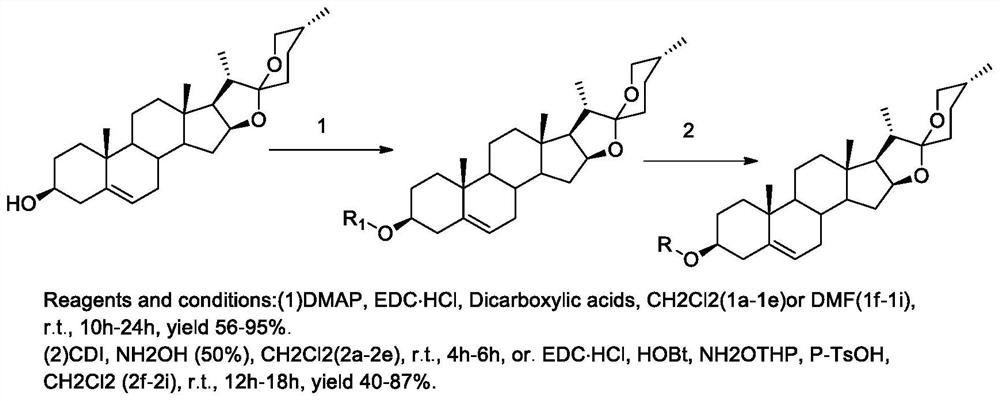
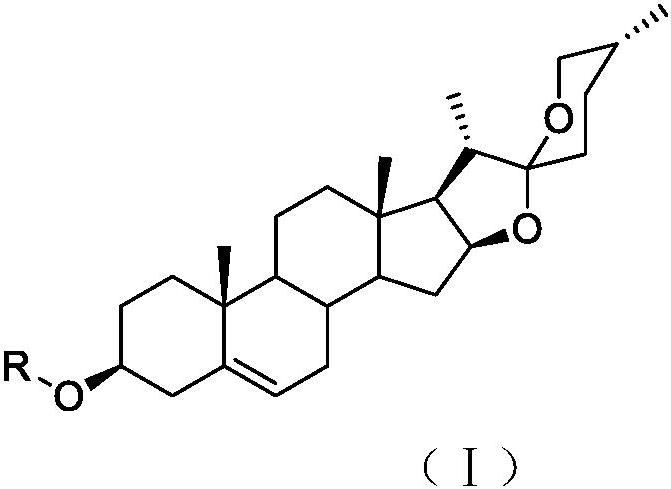
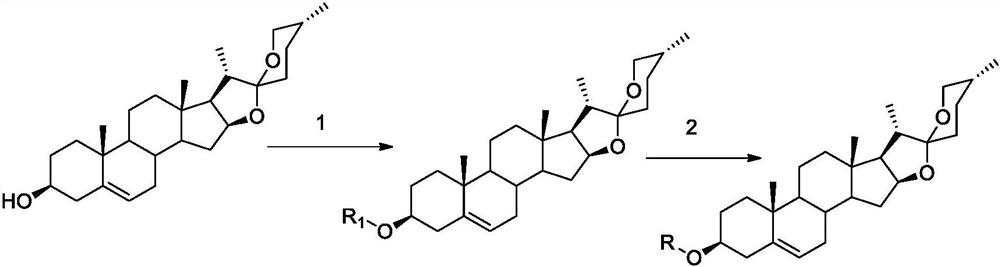
[0038] 5. The preparation method of the compound of general formula (I):
[0039]
[0040] Reagents and conditions: (1) DMAP, EDC·HCl, Dicarboxylic acids, CH2Cl2(1a-le) or DMF(lf-1i), r.t., 10h-24h, yield 56-95%.
[0041](2) CDI, NH2OH (50%), CH2Cl2 (2a-2e), r.t., 4h-6h, or. EDC·HCl, HOBt, NH2OTHP, P-TsOH, CH2Cl2 (2f-2i), r.t., 12h-18h , yield 40-87%.
[0042] Synthesis of Compound 1a_1e
[0043] Dissolve diosgenin (1.0g, 2.41mmol) in dichloromethane (30mL), add EDC·HCl (0.55g, 2.90mmol), 4-dimethylaminopyridine (0.354g, 2.90mmol), succinic anhydride (0.97g, 9.96mmol), react at 25°C for 10h, and TLC detects that the reaction is complete. The reaction solution was washed successively with 2N hydrochloric acid (3×10mL), 2N sodium bicarbonate (3×10mL), and water (3×10mL), the organic layer was dried over anhydrous sodium sulfate, concentrated under reduced pressure to recover dichloromethane, passed through a silica gel column Chromatography (dichloromethane:methanol (v / v)...
Embodiment
[0062] 1. Synthesis of Compound 1
[0063] Synthesis of Compound 1a
[0064] Diosgenin (1.0g, 2.41mmol, 1eq.) was dissolved in dichloromethane (30mL), and EDC·HCl (0.55g, 2.90mmol, 1.2eq.), 4-dimethylaminopyridine (0.354g, 2.90mmol, 1.2eq.), succinic anhydride (0.97g, 9.96mmol, 4eq), reacted at 25°C for 10h, and TLC detected that the reaction was complete. The reaction solution was washed successively with 2N hydrochloric acid (3×10mL), 2N sodium bicarbonate (3×10mL), and water (3×10mL), the organic layer was dried over anhydrous sodium sulfate, concentrated under reduced pressure to recover dichloromethane, passed through a silica gel column Chromatography (dichloromethane:methanol (v / v)=50:1) separated a white solid with a yield of 87%. m.p.222-224℃.1H NMR (400MHz, CDCl3) δ5.36 (d, J = 5.0Hz, 1H, H-6), 4.66-4.58 (m, 1H, H-3), 4.41 (q, J = 7.4Hz,1H,H-16),3.52-3.30(m,2H,H-26),2.70-2.54(m,4H,-COCH2-),2.31(d,J=7.0Hz,2H,H-4 )ppm.13C NMR (100MHz, CDCl3) δ177.36,171.52,139.53,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com