Novel marker for auxiliary diagnosis of valvular heart disease and auxiliary diagnosis product
A technology for assisting diagnosis and heart disease, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Serum collection from healthy volunteers or patients with valvular heart disease
[0019] 1.1 Sample recruitment of healthy volunteers: healthy volunteers were recruited voluntarily and signed informed consent. The number of healthy volunteers included in this study was 27, including 14 males and 13 females. The age distribution was as follows: 8 people were 21-30 years old, 11 people were 31-40 years old, 3 people were 41-50 years old, and 5 people were 51-60 years old.
[0020] 1.2 Collection of patients with valvular heart disease: The patients with valvular heart disease included in this study were admitted to Nanhai Hospital of Guangdong Provincial People's Hospital and completed blood collection. This study was carried out under the condition that the patients were informed and voluntarily signed the informed consent. Among the patients in this study, there were 23 males and 25 females. The age distribution was as follows: 1 patient was 70 years old....
Embodiment 2
[0022] Embodiment 2: Serum NT-ProBNP level detection
[0023] 2.1: Sample preparation: Freshly separated serum can be tested directly; if it is a frozen sample, it needs to be thawed at room temperature and mixed thoroughly by low-speed vortex or inversion.
[0024] 2.2: Detection of NT-ProBNP level: NT-ProBNP level was detected according to the instructions of Brain Natural Peptide N-terminal Precursor Protein Assay Kit (chemiluminescent method) (New Industry, 130206004M).
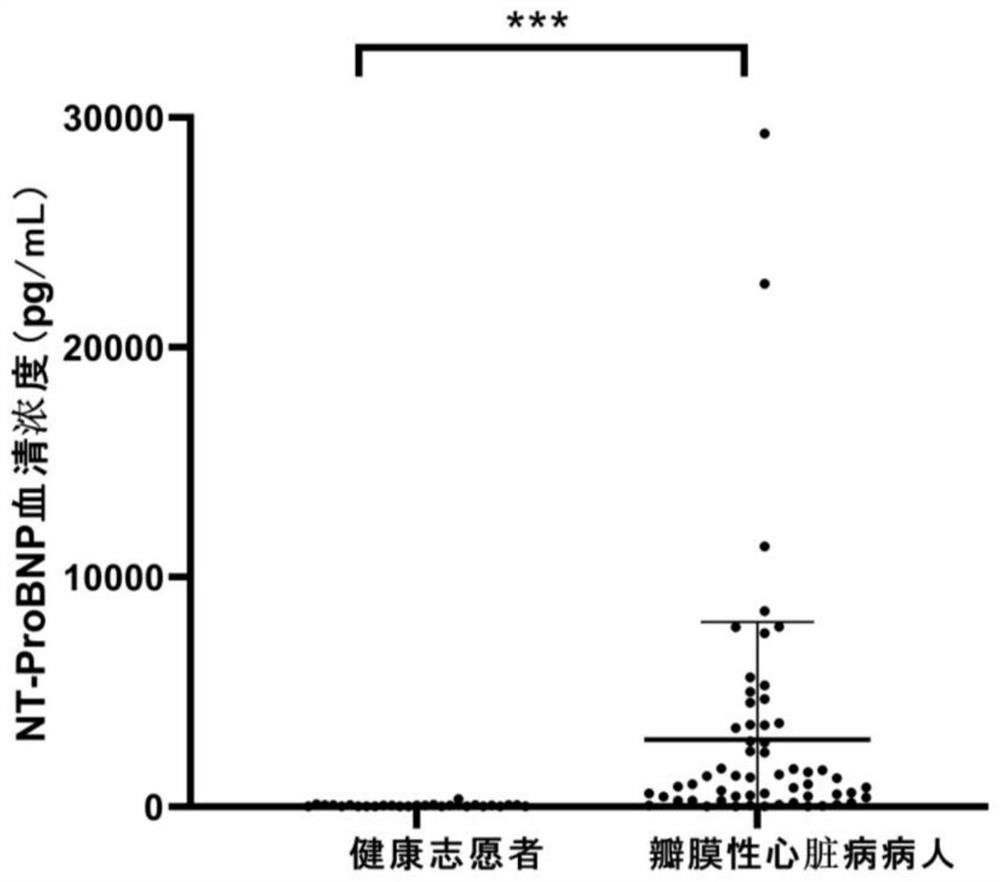
[0025] Result analysis: if figure 2 , as shown in Table 1, the serum NT-ProBNP level of patients with valvular heart disease was significantly higher than that of healthy volunteers, but the NT-ProBNP level of 34.5% (20 / 58) patients still had been diagnosed in the normal range, indicating that the NT-ProBNP level was in the normal range. Sensitivity / accuracy of ProBNP levels as a biomarker of valvular heart disease is suboptimal. Therefore, it is not comprehensive to use only NT-ProBNP as a biomarker i...
Embodiment 3
[0026] Example 3: Detection of serum hsCRP level
[0027] 3.1: Sample preparation: Freshly separated serum can be tested directly; if it is a frozen sample, it needs to be thawed at room temperature and mixed thoroughly by low-speed vortex or inversion.
[0028] 3.2: Detection of hsCRP level: The hsCRP level was detected according to the instructions of the C-reactive protein assay kit (chemiluminescent method) (New Industry, 130216002M).
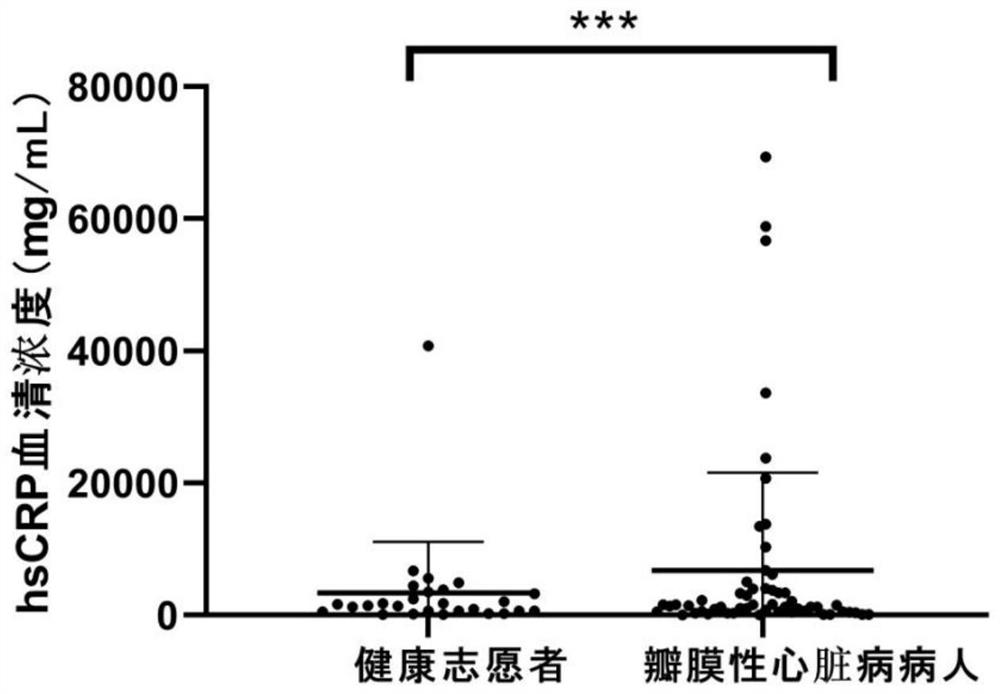
[0029] Result analysis: if image 3 , as shown in Table 1, 31.0% (18 / 58) of serum hsCRP levels in patients with confirmed valvular heart disease are displayed in the normal range, indicating that the sensitivity / accuracy of using hsCRP levels as biomarkers of valvular heart disease not ideal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com