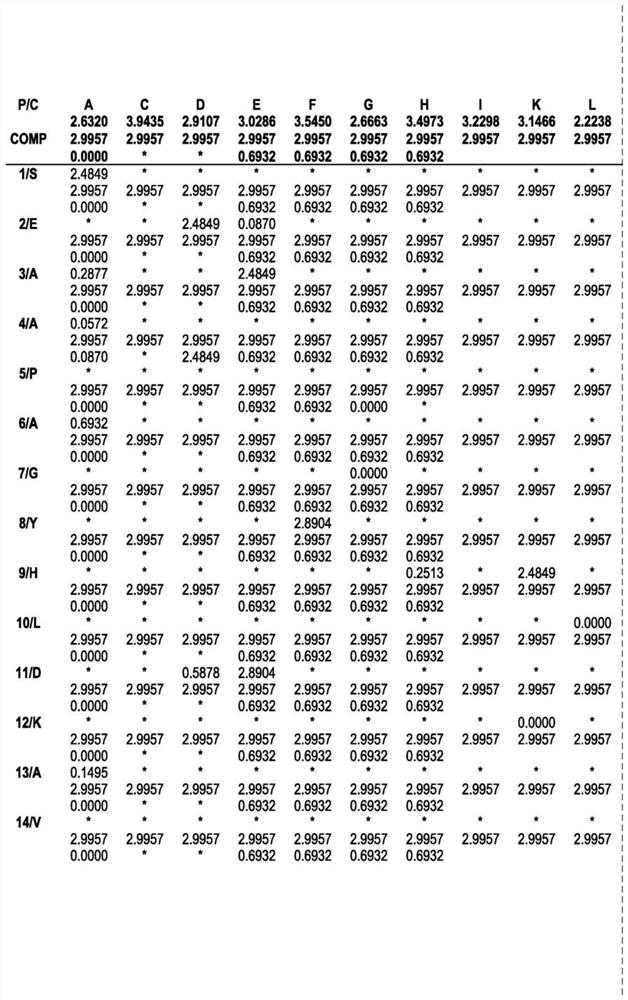
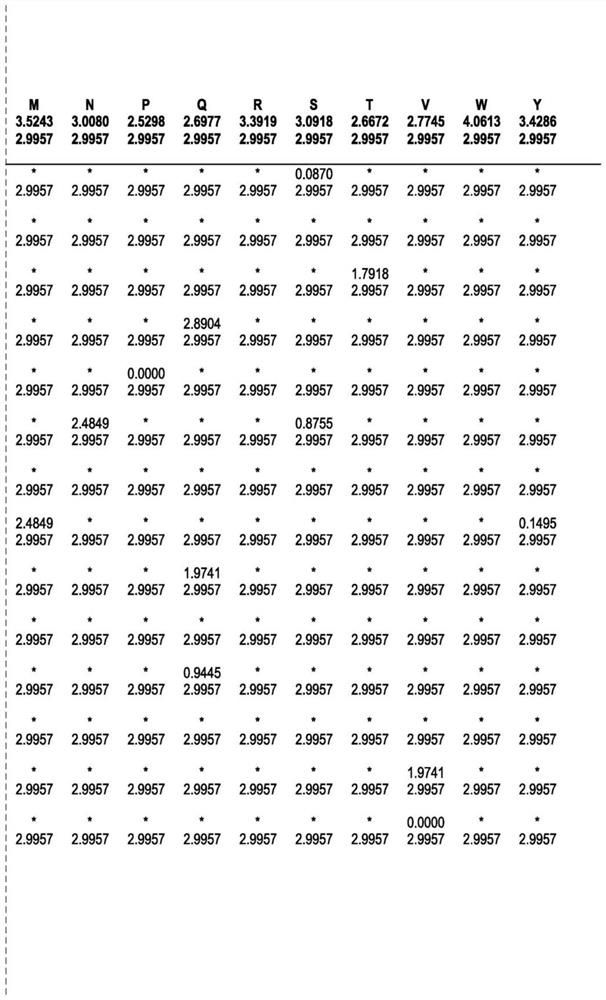
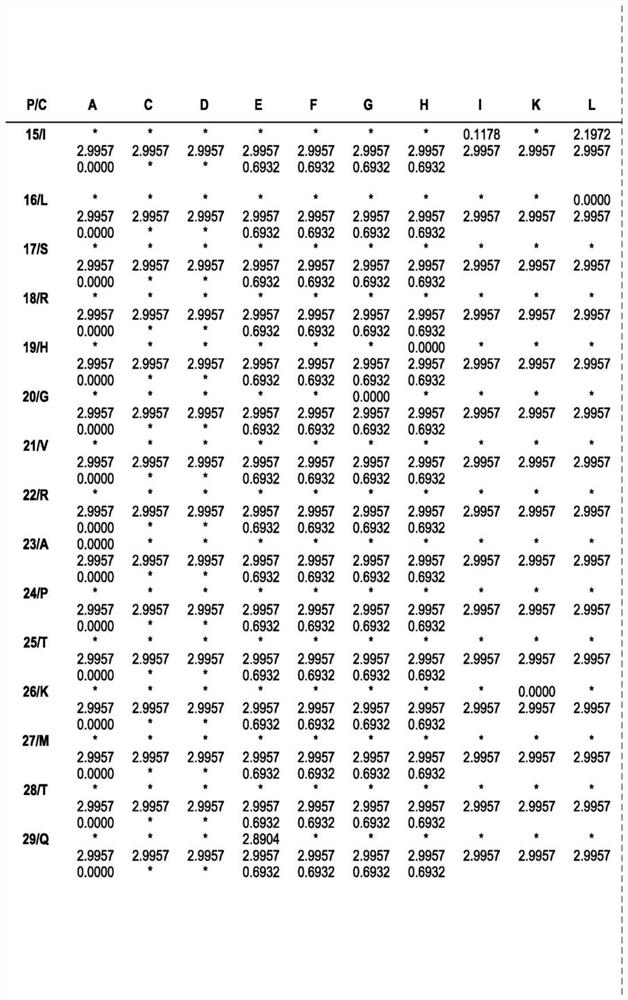
ENGINEERED ROBUST HIGH Tm-PHYTASE CLADE POLYPEPTIDES AND FRAGMENTS THEREOF
A phytase, engineering technology, applied in the direction of enzymes, hydrolases, biochemical equipment and methods, etc., can solve problems such as lack of robustness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0462] Unless defined otherwise herein, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this disclosure belongs. Singleton et al., DICTIONARY OFMICROBIOLOGY AND MOLECULAR BIOLOGY, 2nd ed., John Wiley and Sons, New York (1994), and Hale and Marham, THE HARPER COLLINSDICTIONARY OF BIOLOGY [ HarperCollins Biological Dictionary], Harper Perennial, New York (1991) provides the skilled artisan with a general dictionary of many of the terms used in this disclosure.
[0463] The disclosure is further defined in the examples below. It should be understood that these Examples, while indicating certain embodiments, are given by way of illustration only. From the above discussion and these Examples, one skilled in the art can ascertain the essential characteristics of this disclosure, and without departing from the spirit and scope of this disclosure, can make various changes and modifications to ada...
example 1
[0465] Production of Phytase Molecule
[0466] DNA manipulations were performed using molecular biology techniques known in the art to generate the phytase gene. Polynucleotide fragments corresponding to the coding sequences of various phytases were synthesized using preferred codons of the fungal expression host Trichoderma reseei (T. reesei), and randomly reassembled using PCR technology. The signal sequence of the pepl aspartic protease from Trichoderma reesei (SEQ ID NO: 63), artificially interrupted by the pepl intron, was introduced into the N-terminus (5' end) of each phytase gene sequence. As recommended by the supplier, use BP recombinant technology introduced the gene into pDonor221 vector (Invitrogen, USA). The resulting entry plasmid (entry plasmid) was recombined with the destination vector pTTTpyr2 to obtain the final expression vector. pTTTpyr2 is similar to the previously described pTTTpyrG vector (PCT Publication WO 2011 / 063308), except that the pyrG gene ...
example 2
[0470] Preparation and Characterization of Phytase
[0471] Protein Purification and Standardization
[0472]The T. reesei strain encoding the recombinant phytase was grown as described above, and the clarified supernatant was used to purify the phytase. The filtered culture supernatant was diluted 5-fold with wash buffer (25 mM sodium acetate, pH 5.5), and loaded onto an MTP filter plate (Millipore (Millipore) Multiscreen Solvinert deep-well filter plate 96-well MTP, 0.45 uM Aqueous membrane, #MDRLN0410) on cation exchange resin (WorkBeads 40S from Bio-Works) equilibrated with purified water. The MTP was placed in a centrifuge and the effluent was discarded during the 1 minute centrifugation (100 x g). Phytase protein samples were eluted using elution buffer (25 mM sodium acetate, 0.5 M NaCl, pH 5.5) during 1 min of centrifugation (100 x g). Samples from the protein purification step were diluted 5-fold with sodium acetate buffer (25 mM sodium acetate, 0.5 M NaCl, pH 5.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| water activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com