A kind of bioactive preparation based on tumor cell membrane and its preparation method and application
A bioactive, cell membrane technology, applied in the field of medicine, to achieve good biocompatibility, excellent anti-tumor effect, and improve the effect of anti-tumor effect in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] In yet another specific embodiment of the present invention, a method for preparing the above-mentioned cell membrane-based bioactive preparation is provided, comprising:
[0061] The tumor cell membrane modified by sodium alginate oxide forms a gelling factor. The CDK5 inhibitor and the exosome inhibitory drug are respectively dissolved and mixed uniformly to form a drug solution, which is added to the aqueous solution of the gelling factor and stirred to obtain a gelling solution.
[0062] In another specific embodiment of the present invention, the solvent for dissolving the CDK5 inhibitor and the exosome inhibitor is deionized water, which has a better dissolving effect, and the method of adding the drug solution is dropwise, so that the antitumor drug components are dispersed more efficiently. uniform;
[0063] In another specific embodiment of the present invention, the cell membrane vesicles are prepared by the following method:
[0064] (1) Collect mouse tumor ...
Embodiment 1
[0095] Example 1: Preparation of sodium alginate oxide-modified tumor cell membrane vesicles
[0096] 1) Preparation of tumor single cell suspension: After collecting mouse melanoma tissue, it was obtained by adding homogenization buffer containing 0.25mM sucrose, 1mM EDTA, 20mM HEPES-NaOH and protease inhibitor agent in PBS buffer.
[0097] 2) Extraction of tumor cell membranes: The collected tumor single cell suspension was subjected to probe ultrasound at 4°C to break the tumor cells to obtain broken tumor cells. Off: 2s; centrifuge the broken tumor cells at 3000g for 10min at 4°C to remove melanin for purification; centrifuge the purified broken cell suspension at 15000g for 60min at 4°C to obtain tumor cell membrane pellets; redisperse the cell membrane pellets in PBS, cryopreserved.
[0098] 3) Preparation of tumor cell membrane vesicles: The tumor cell membrane was sonicated at 4° C. for 1 min to finally obtain tumor cell membrane vesicles.
[0099]4) Preparation of ...
Embodiment 2
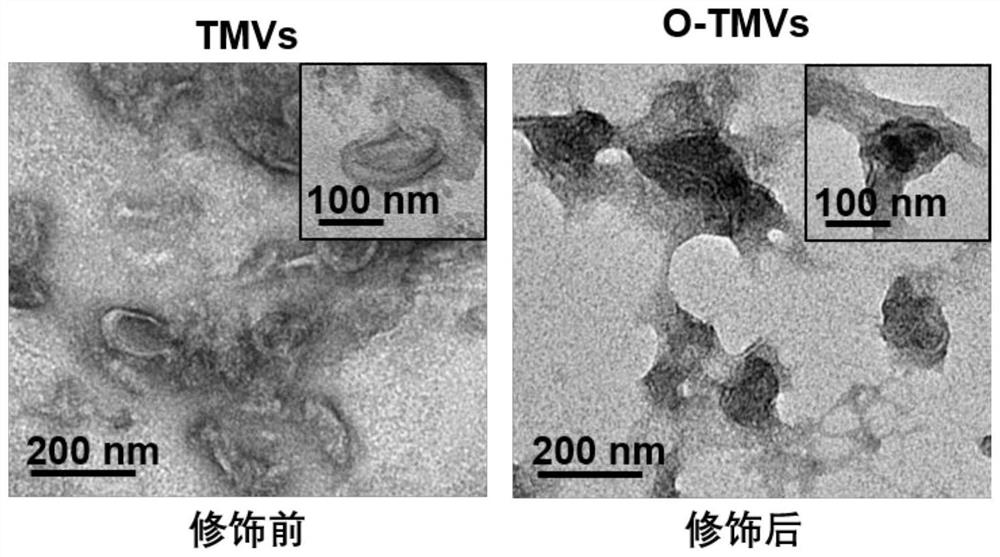
[0101] Example 2: Transmission electron microscopy (TEM) identification of nanostructures of tumor cell membrane vesicles before and after modification
[0102] Pipette 10 μL of tumor cell membrane vesicle solution before and after modification, respectively, absorb excess liquid on carbon membrane copper mesh, filter paper, dry at room temperature and observe under transmission electron microscope. The result is as figure 1 As shown, transmission electron microscopy images can confirm that the morphology of tumor cell membrane vesicles before and after modification is significantly different, with sizes of ~160 and ~200 nm, respectively, with uniform size and good dispersion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com