Preparation method and application of interfering peptide targeting SARS-CoV-2 N protein
A sars-cov-2n and protein technology, applied in the field of preparation of interfering peptides, can solve the problems of limited plasma sources and inability to use on a large scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis and detection of interfering peptide drug NIP-V
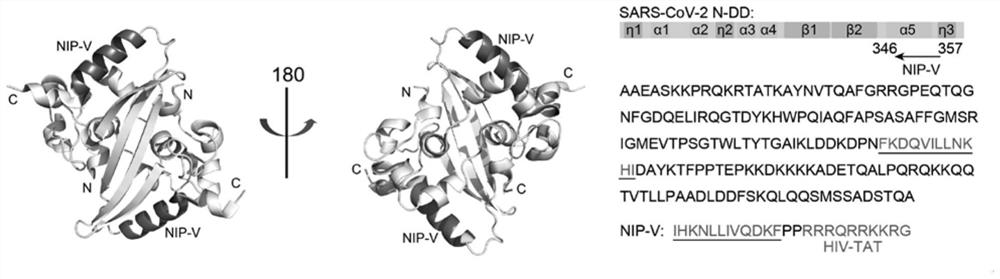
[0042] The NIP-V amino acid sequence of the interference peptide drug designed by the present invention is IHKNLLIVQDKFPPRRRQRRKKRG, and the targeting sequence is as follows: figure 1 As indicated, D-amino acid was used as a raw material to synthesize at Jill Biochemical (Shanghai) Co., Ltd.
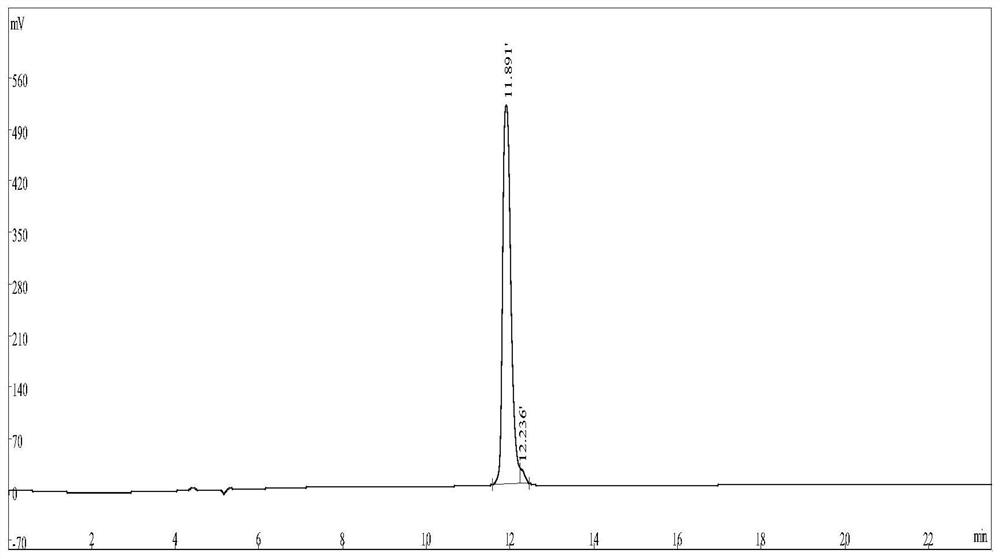
[0043] Such as figure 2 As shown, the synthetic interfering peptide drug NIP-V is identified by the Agilent-6125B liquid mass spectrometry system (Agilent Technologies) as having a molecular weight of 3040.69, and the HPLC adopts an Inertsil ODS-SP liquid chromatography column (Shimadzu, 4.6mm × 250mm) as a stationary phase , using mobile phase A (100% nitrile, 0.1% trifluoroacetic acid) and mobile phase B (100% ultrapure water, 0.1% trifluoroacetic acid) for gradient elution, such as image 3 And shown in Table 1, through HPLC identification, purity is greater than 98%.
[0044] keep time content(%) Pea...
Embodiment 2
[0047] Treatment of ACE2 transgenic mice with the interfering peptide drug NIP-V can significantly reduce the proliferation of SARS-CoV-2 in mice.
[0048] 1 Experimental materials
[0049] ACE2 transgenic mice, Daan gene novel coronavirus (2019-nCoV) nucleic acid detection kit (fluorescent PCR method), SARS-CoV-2, NIP-V interfering peptide medicine prepared in Example 1.
[0050] 2 Experimental methods
[0051] Use sterilized PBS to dissolve the NIP-V interfering peptide drug to a concentration of 1 mg / mL. ACE2 transgenic mice were divided into 4 groups with 8 mice in each group. The first and third groups were injected with 0.5 mL sterilized PBS as a control, and the second and fourth groups were injected with 0.5 mL (0.5 mg) NIP-V drug. One hour later, all four groups of mice were anesthetized and intranasally inoculated with SARS-CoV-2, each mouse was inoculated with approximately 1×105 TCID50 virus. After 16 hours and 24 hours of virus infection, the lung tissues of th...
Embodiment 3
[0059] Treatment of ACE2 transgenic mice with the interfering peptide drug NIP-V can significantly inhibit the lung lesions caused by SARS-CoV-2 infection.
[0060] 1 Experimental materials
[0061] ACE2 transgenic mice, SARS-CoV-2, the NIP-V interfering peptide drug prepared in Example 1. Reagents related to tissue fixation, embedding and HE staining (Shenggong).
[0062] 2 Experimental methods
[0063] Use sterilized PBS to dissolve the NIP-V interfering peptide drug to a concentration of 1 mg / mL. ACE2 transgenic mice were divided into 3 groups, the first group was not infected, the second group was injected with 0.5 mL sterilized PBS, and 1 hour later intranasally inoculated with 1×10 5 TCID50 of SARS-CoV-2, the third group was injected with 0.5 mg of NIP-V drug, and 1 hour later intranasally inoculated 1×10 5 TCID50 of SARS-CoV-2. After 24 hours of virus infection, the mouse lung tissue was taken, placed in 4% paraformaldehyde / PBS for tissue fixation, and paraffin sec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com