Leucocyte immunoglobulin-like receptor neutralizing antibodies
A technology of cytotoxicity and antibodies, applied in the direction of immunoglobulin, anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve unmet problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
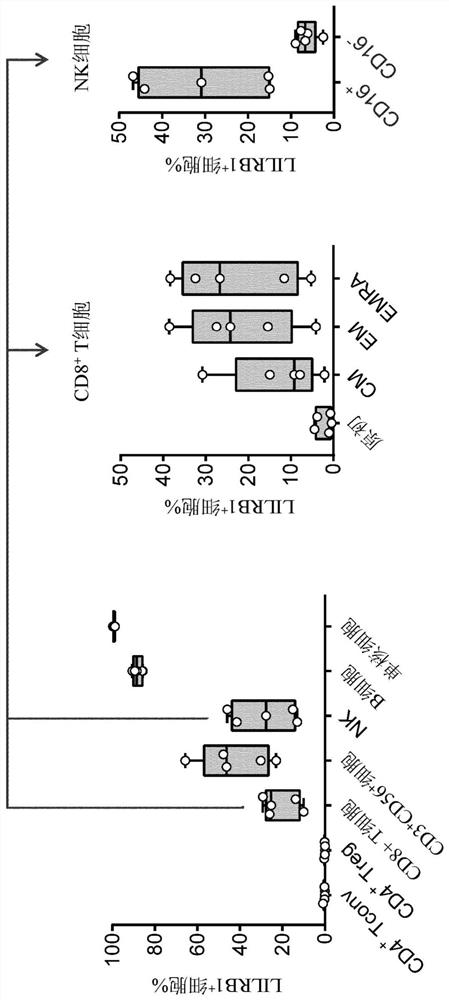
[0313] Example 1: ILT2 (LILRB1) in healthy human donor and memory CD8 T cells expressed the CD56 dim NK cells
[0314] LILRB1 determined expression on peripheral blood mononuclear cells by fresh whole blood from healthy human donors were subjected to flow cytometry. NK group was determined to be CD3-CD56 + cells (anti-CD3 AF700- biological legendary company (BioLegend) # 300424; anti-CD56 BV421-BD Biosciences (BD Biosciences) # 740076). In NK cells, CD56 bright subsets were identified as CD16- cells and CD56 dim subsets were identified as CD16 + cells (anti CD16BV650-BD Biosciences # 563691). CD4 + and CD8 + T cells were identified as CD3 + CD56-CD4 + and CD3 + CD56-CD8 + cells (CD3- supra; CD4 BV510-BD Biosciences # 740161; CD8BUV737-BD Biosciences # 564629). In CD4 + T cell population, Tconv and Treg were identified as CD127 + CD25- / low CD127 CD25 high and low cells (CD127 PE-Cy7-BD Biosciences # 560822; CD25VioBright- Miltenyi Biotech (Miltenyi Biotec) # 130-104-274). In CD8 ...
example 2
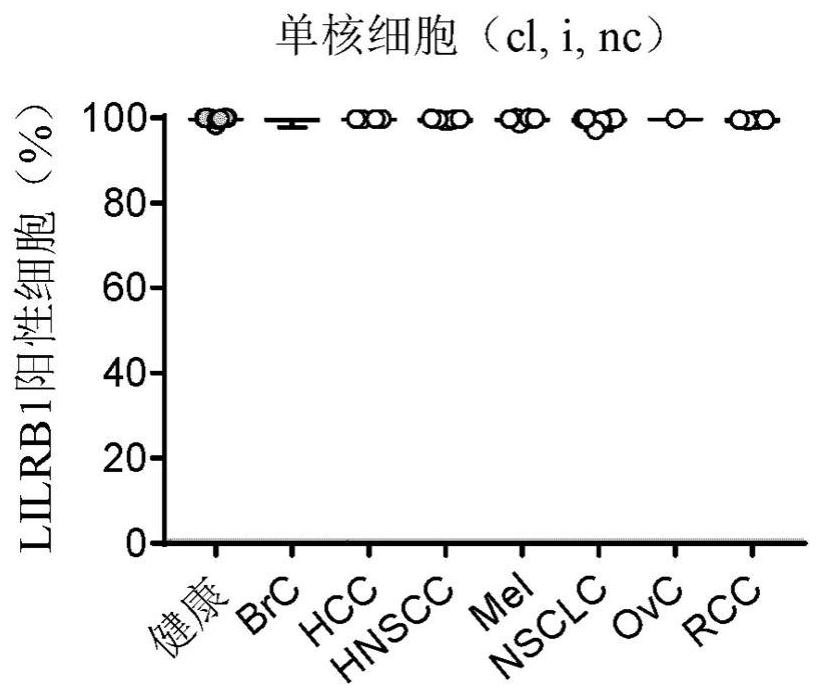
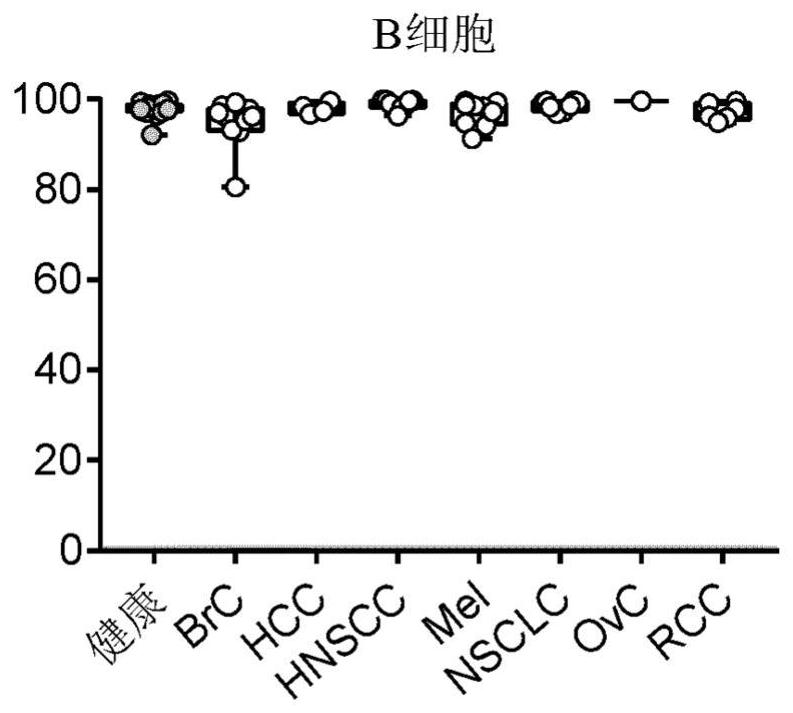
[0317] Examples 2: ILT2 is upregulated in many human cancers
[0318] Determined by monocytes purified from whole blood of a human cancer patient donor peripheral blood mononuclear cells (PBMC) for flow cytometry, B cells, CD4 + T cells, CD8 + T cells and CD16- and CD16 + ILT2 on NK cells, express both. Using the same antibody as detailed in Example 1 a mixture of cell populations to identify and assess ILT2 expression. PBMC were incubated with the antibody mixture was incubated in the dark at 4 ℃ together for 20 minutes, washed twice in staining buffer, fluorescence was measured by flow cytometry on Fortessa.
[0319] Results from a sample of a cancer patient is shown in FIG. As can be seen, ILT2 again is expressed on all B cells and monocytes. However, in lymphocyte subsets, NK cells and CD8 T cells, on the cell of ILT2 from three types of cancer (HNSCC, NSCLC and RCC) more frequently (statistically significant) is expressed. ILT2 also up-regulated in ovarian cancer, the patient...
example 3
[0320] Example 3: generating an anti-antibody ILT2
[0321] Materials and Methods
[0322] ILT-2_6xHis clone and produce recombinant proteins
[0323] ILT-2 protein (the Uniprot accession number Q8NHL6) between NruI restriction site and BamHI restriction site was cloned into the vector pTT-5. Use of the heavy chain leader peptide. PCR was performed using the following primers:
[0324] ILT-2_ Forward _ACAGGCGTGCATTCGGGGCACCTCCCCAAGCCCAC (SEQ ID NO: 57)
[0325] ILT-2_ reverse _CGAGGTCGGGGGATCCTCAATGGTGGTGATGATGGTGGTGCCTTCCCAGACCACTCTG (SEQ ID NO: 58)
[0326] Added 6xHis tag at the C-terminal portion of the protein for purification. Transfected with the vector generated EXPI293 cell lines for transient production. Protein was purified from the supernatant using Ni-NTA beads, and purified using SEC monomer.
[0327] The amino acid sequence of ILT-2_6xHis recombinant protein as follows:
[0328] GHLPKPTLWAEPGSVITQGSPVTLRCQGGQETQEYRLYREKKTALWITRIPQELVKKGQFPIPSITWEHAGRYRCYYGSDTAGRSESS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com