Process for preparing vaccine compositions
A composition and drug technology, applied in the field of vaccine composition preparation, can solve the problems of low ratio of anti-tumor T cell response, no proven efficacy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
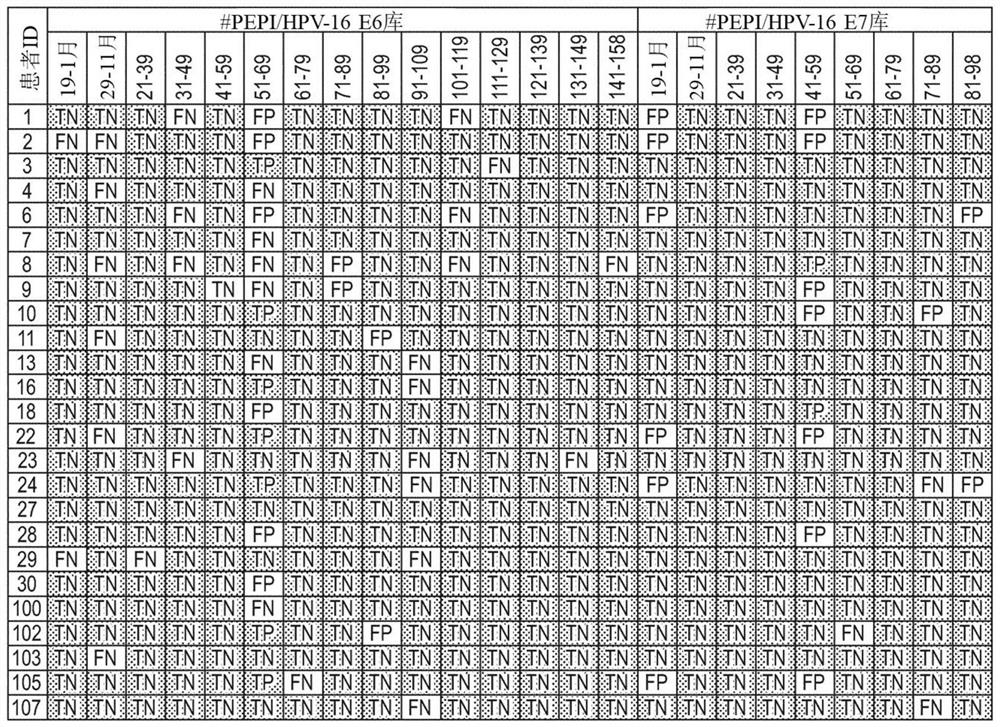
[0333] Example 1 HLA epitope binding prediction method and verification
[0334] Prediction of binding between specific HLAs and epitopes (9mer peptides) was based on the ImmunoEpitope Database tool for epitope prediction (www.iedb.org).
[0335] The HLA I epitope binding prediction process was validated by comparison with HLA I epitope pairs determined by laboratory experiments. Datasets of HLA I epitope pairs reported in peer-reviewed publications or public immunology databases were compiled.
[0336] The rate of agreement with the experimentally determined data set (Table 6) was determined. Binding HLA I epitope pairs for the dataset were correctly predicted with 93% probability. Coincidentally, non-binding HLA I epitope pairs were also predicted correctly with a probability of 93%
[0337] Table 6 Analytical specificity and sensitivity of HLA epitope binding prediction methods
[0338]
[0339] The accuracy of predicting multiple HLA binding epitopes was also dete...
example 2
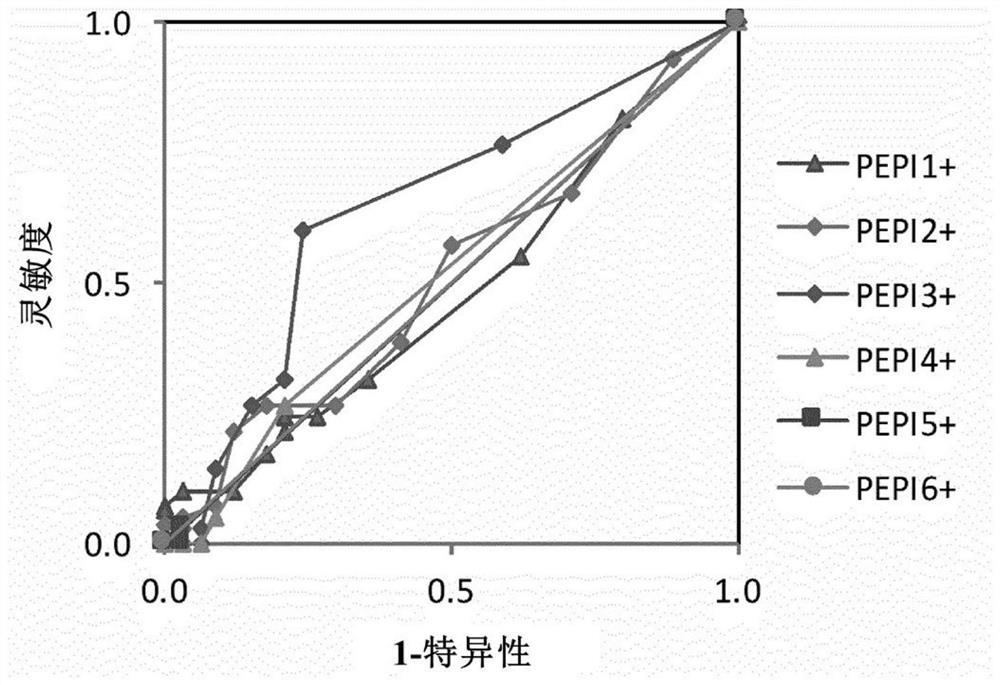
[0343] Example 2 Epitope Presentation of Multiple HLAs Predicts Cytotoxic T Lymphocyte (CTL) Response
[0344] This study investigated whether an individual's presentation of one or more epitopes of a polypeptide antigen by one or more HLA class I molecules is predictive of a CTL response.
[0345] The study was performed by a retrospective analysis of 6 clinical trials conducted in 71 cancer patients and 9 HIV-infected patients (Table 8). Patients from these studies were treated with HPV vaccines, three different NY-ESO-1-specific cancer vaccines, an HIV-1 vaccine, and a CTLA-4-specific monoclonal antibody (ipilimmab), the Antibodies shown to reactivate CTLs against NY-ESO-1 antigen in melanoma patients. All of these clinical trials measure antigen-specific CD8+ CTL responses (immunogenicity) in study subjects after vaccination. In some cases, a correlation between CTL response and clinical response was reported.
[0346] No patients were excluded from the retrospective ...
example 4
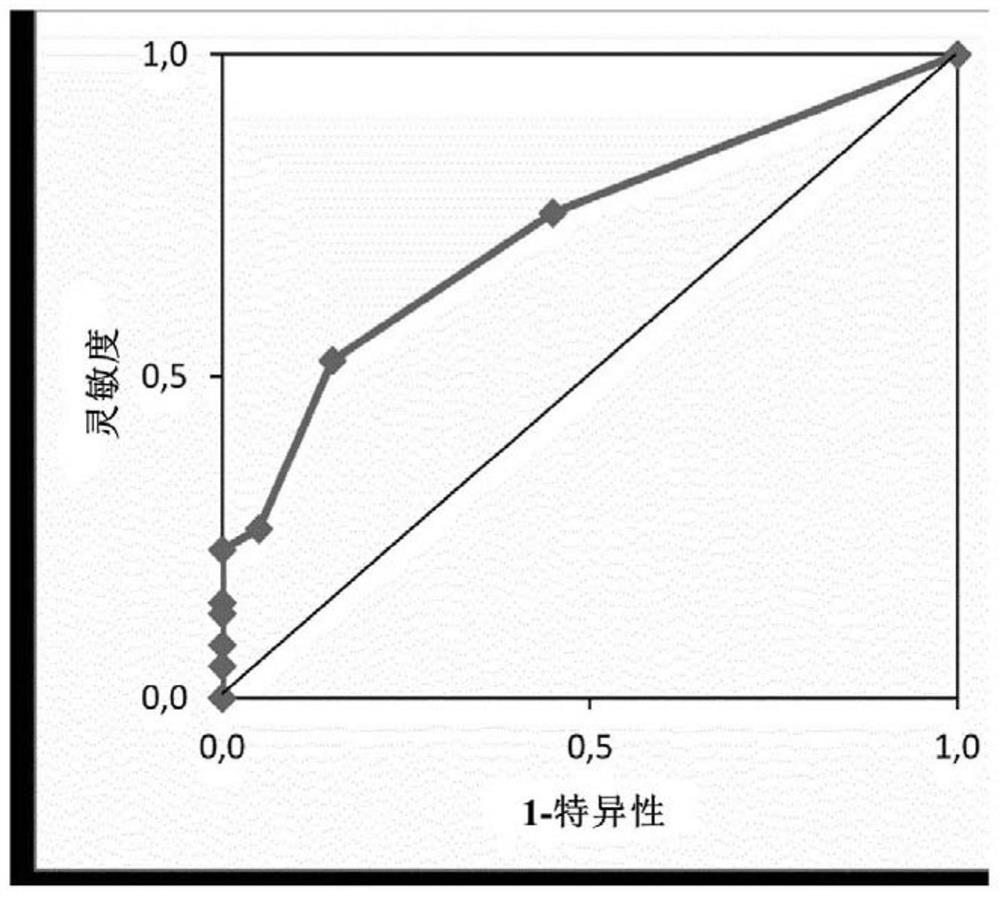
[0367] Example 4 Clinical Validation of PEPI3+ Threshold as a New Biomarker for PEPI Test
[0368] Vaccine design based on PEPI3+ biomarkers has been tested for the first time in a phase I clinical trial in patients with metastatic colorectal cancer (mCRC) in the OBERTO phase I / II clinical trial (NCT03391232). In this study, we evaluated the safety, tolerability, and immunogenicity of single or multiple doses of PolyPEPI1018 as an add-on to maintenance therapy in patients with mCRC. PolyPEPI1018 is a peptide vaccine containing 12 unique epitopes from 7 conserved TSAs frequently expressed in mCRC (WO2018158455A1). These epitopes are designed to bind at least three autologous HLA alleles that are more likely to induce T cell responses than epitopes presented by a single HLA (see Examples 2 and 3). mCRC patients in the first-line setting received the vaccine (dose: 0.2 mg / peptide) immediately after transition to maintenance therapy with fluoropyrimidine and bevacizumab. Vacci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com