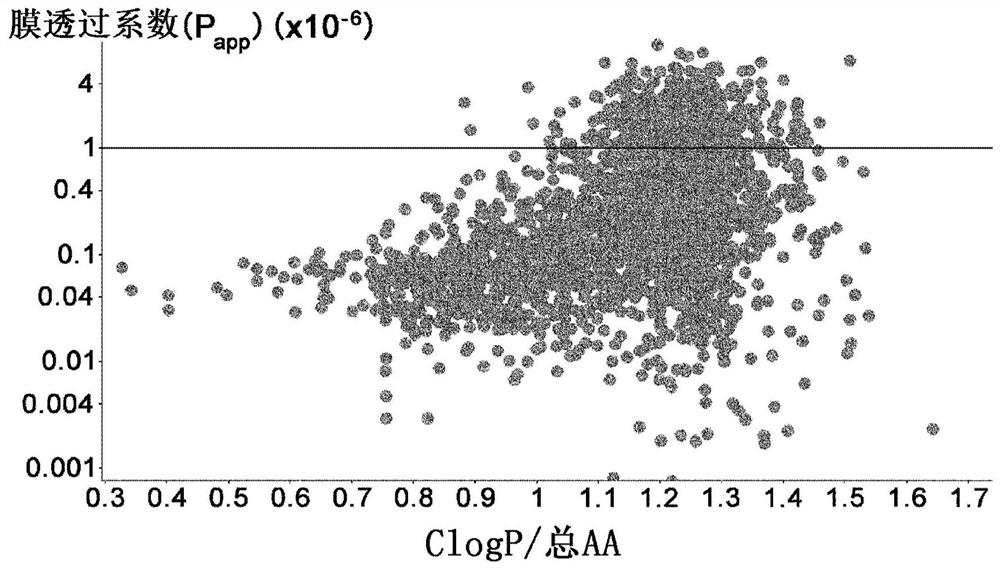
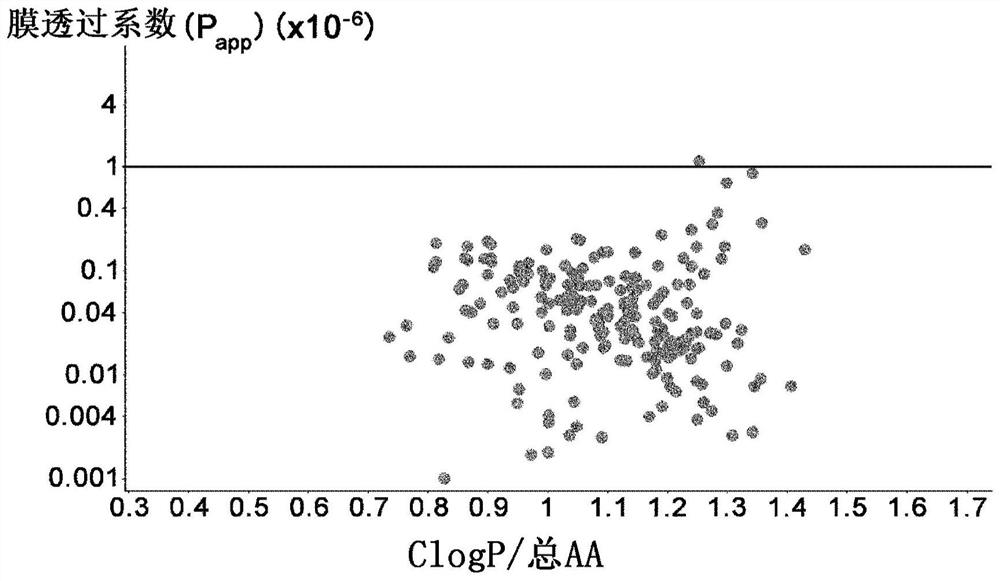
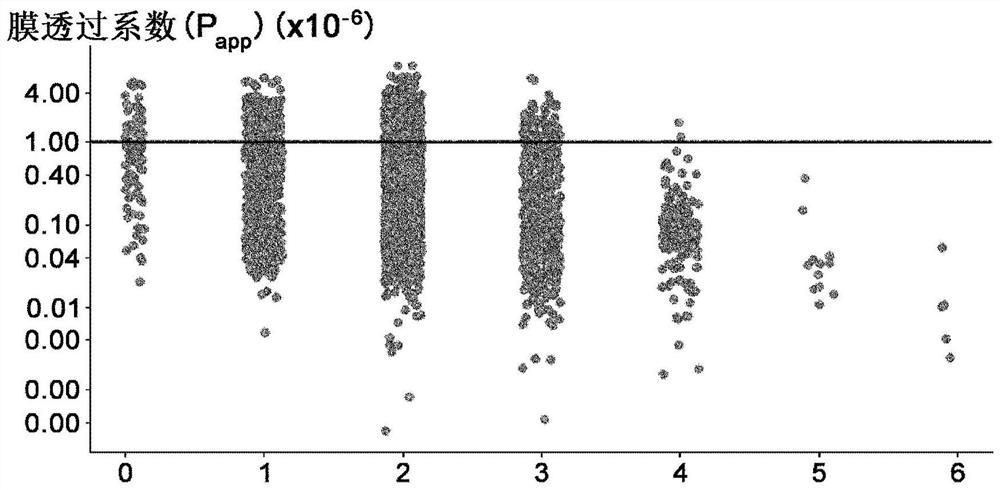
Amino acid having functional group capable of intermolecular hydrogen bonding, peptide compound containing same and method for production thereof
A technology of peptide compounds and amino acids, applied in the field of amino acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0306] (usual preparation method)
[0307] The general production method of the amino acid capable of forming a hydrogen bond in the side chain and the peptide compound of the present invention will be described below.
[0308] (Method for preparing amino acid capable of forming intramolecular hydrogen bond)
[0309] In the production of amino acids having intramolecular hydrogen bonds in their side chains, amino acids having ether bonds in their side chains can be produced by the following two synthesis methods.
[0310] Method 1:
[0311] In the presence of a Lewis acid, the aziridine compound having a protected amino group and a carboxylic acid group is reacted with an alcohol having a corresponding suitable protecting group to carry out a ring-opening reaction, followed by deprotection of an undesired protecting group. In the following general formula, it can be used as P 1 Use Cbz group, Fmoc group, Alloc group, nosyl group (Ns group), etc., as P 2 Me group, allyl gro...
Embodiment 1
[0409] The chemical synthesis of embodiment 1 peptide compound
[0410] According to the peptide synthesis method based on the Fmoc method described in WO2013 / 100132, the peptide was elongated in the following basic route. That is, a five-step process: 1) a peptide elongation reaction by the Fmoc method starting from the N-terminal of Asp in which the carboxylic acid of the side chain of Asp is supported on 2-chlorotrityl resin, 2) from The process of cleavage of peptide by 2-chlorotrityl resin, 3) amide cyclization, which is based on the carboxylic acid of the Asp side chain generated from the cleavage process from the 2-chlorotrityl resin and the peptide chain N-terminal (triangle unit), 4) deprotection of the protective group of the side chain functional group contained in the peptide chain, 5) purification of the compound based on preparative HPLC. In addition, in the peptide compound containing an amino acid having an acidic functional group in the side chain, in order t...
Embodiment 2
[1183] Example 2 Membrane Permeability Evaluation of Amino Acids Having a Donor in the Side Chain and an Intramolecular Hydrogen Bond in the Donor Moiety
[1184] 2-1 Comparison of membrane permeability of those having an intramolecular hydrogen bond at the donor moiety, and those not having, among amino acids having a donor at the side chain
[1185] In order to confirm that among amino acids with donors in their side chains, those with intramolecular hydrogen bonds in the donor moiety have better membrane permeability than those without, 2 cyclic peptides containing each amino acid were synthesized by The improved method described in International Publication No. 2018 / 124162 compares membrane permeability. Several combinations of the two cyclic peptides described above were synthesized, and the generality was confirmed by evaluation. As a result, it was confirmed that in (1) Ser(EtOH) and Nle(6-OH), (2) Ser(1-CF3-EtOH) and Hnl(7-F3-6-OH), (3) Tyr(3- OMe) and Tyr, (4) combi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com