Pipeline continuous fluorination method using fluorine salt as fluorine source
A pipeline-based, fluorine-salt technology, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as high safety risks, large size, and low production efficiency, and achieve high reaction yield, simple operation, The effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
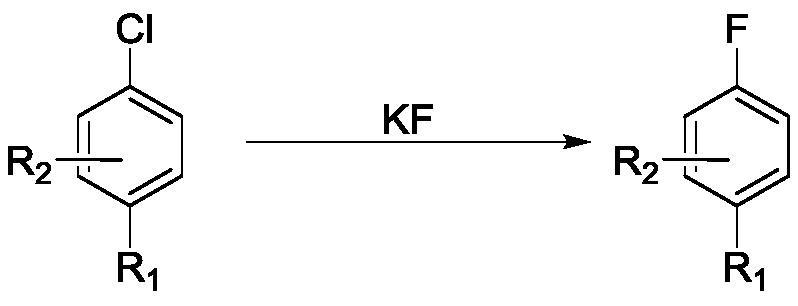
[0056] Embodiment 1-1, a method for preparing aryl (heterocyclic) fluorides, comprising the steps of:
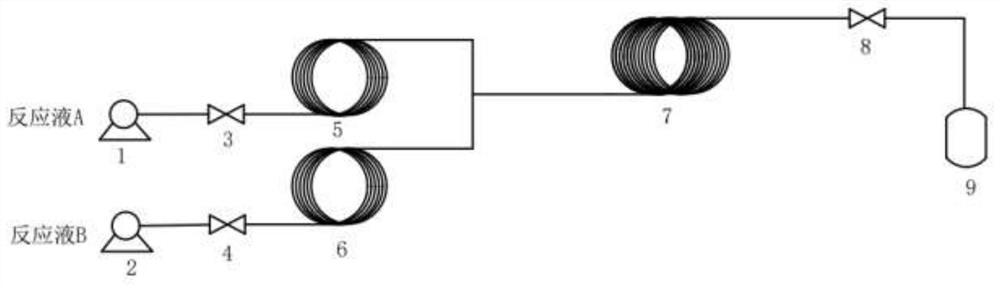
[0057] Reaction solution A was prepared from 22mL water, 23mL dimethyl sulfoxide, 8.82g potassium fluoride, stirred and dissolved at room temperature, and the mass content of water in reaction solution A was about 39.2%; reaction solution B was prepared from 200mL dimethylsulfoxide Sulfoxide, 18.27g p-chloronitrobenzene, stirred and dissolved at room temperature.
[0058] The reaction solution A was pumped into the 1# preheating coil 5 through the 1# metering pump 1 at a flow rate of 1 mL / min, the temperature of the 1# preheating coil 5 was 130° C., and the residence time was 1 min. The reaction solution B was pumped into the 2# preheating coil 6 through the 2# metering pump 2 at a flow rate of 5 mL / min. The temperature of the 2# preheating coil 6 was 130° C., and the residence time was 1 min. The preheated reaction solution A and reaction solution B flow into the reaction ...
Embodiment 1-2
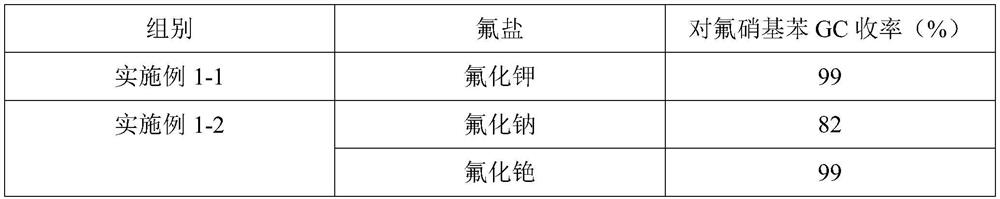
[0063] Change potassium fluoride into sodium fluoride or cesium fluoride respectively, and the molar weight of sodium fluoride or cesium fluoride in reaction solution A is the same as potassium fluoride in embodiment 1-1, control sodium fluoride or fluorine Cesium chloride: p-chloronitrobenzene = 1.1:1 molar ratio. The rest are identical to Example 1-1. The obtained results are shown in Table 1 below.
[0064] Table 1, the impact of fluorine salt on the reaction
[0065]
[0066] Potassium fluoride and cesium fluoride have relatively good reaction effects, but potassium fluoride has a high atomic utilization rate and is cheap, so potassium fluoride is a preferred fluoride salt.
Embodiment 1-3
[0068] The temperature of the reaction coil 7 was changed to 170° C., 190° C., and 200° C., and the other conditions were the same as in Example 1-1. The results obtained are shown in Table 2 below.
[0069] Table 2, the influence of reaction temperature on reaction
[0070]
[0071] The reaction temperature is 170°C, and the yield decreases; while the reaction temperature is 190°C and 200°C, the reaction yield is not as good as the reaction temperature 180°C, because the reaction temperature is too high and by-products are formed, so 180°C is the preferred reaction temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com