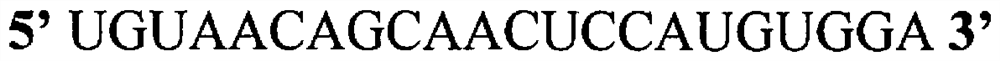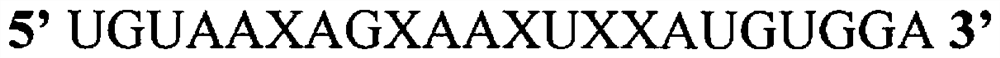Modified micrornas and their use in the treatment of cancer
A cancer, pancreatic cancer technology, applied in the field of modified microRNA and their use in the treatment of cancer, can solve the problem of toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Example 1. Materials and methods.
[0120] modified microRNA . All modified microRNAs were synthesized by automated oligonucleotide synthesis and purified by HPLC. The two strands are annealed to produce a mature modified 5-FU-miR and / or modified miR-194 of the disclosure having cytosine bases replaced by gemcitabine molecules. For the modified microRNA-194 containing 5-halouracil, a method called "2'-ACE RNA synthesis" was used. 2'-ACE RNA synthesis is based on a protecting group scheme using a silyl ether to protect the 5'-hydroxyl group combined with an acid-labile orthoester protecting group (2'-ACE) on the 2'-hydroxyl. This combination of protecting groups is then used with standard phosphoramidate solid phase synthesis techniques. See, eg, S.A. Scaringe, F.E. Wincott, and M.H. Caruthers, J. Am. Chem. Soc. , 120 (45), 11820-11821 (1998); International PCT Application WO / 1996 / 041809; M.D. Matteucci, M.H. Caruthers, J. Am. Chem. Soc. , 103, 3185-3191 (1981); ...
Embodiment 2
[0129] Example 2: Modified miR-194 nucleic acid has anticancer activity.
[0130] In the following experiments, all 5 cytosine bases in the guide strand of native miR-194 (SEQ ID NO:1) were replaced by gemcitabine to form the exemplary modified microRNA shown in SEQ ID NO:2 . see Figure 1C . Another modified microRNA was formed by replacing all uracil bases in the guide strand of the native miR-194 nucleic acid with a 5-FU molecule, as shown in SEQ ID NO. 4. see Figure 1B . In one experiment, all U bases in miR-194 were replaced by 5-FU, and all cytosines (C bases) were replaced by gemcitabine molecules, as in Figure 1D As shown in the provided structure and as shown in SEQ ID NO: 3.
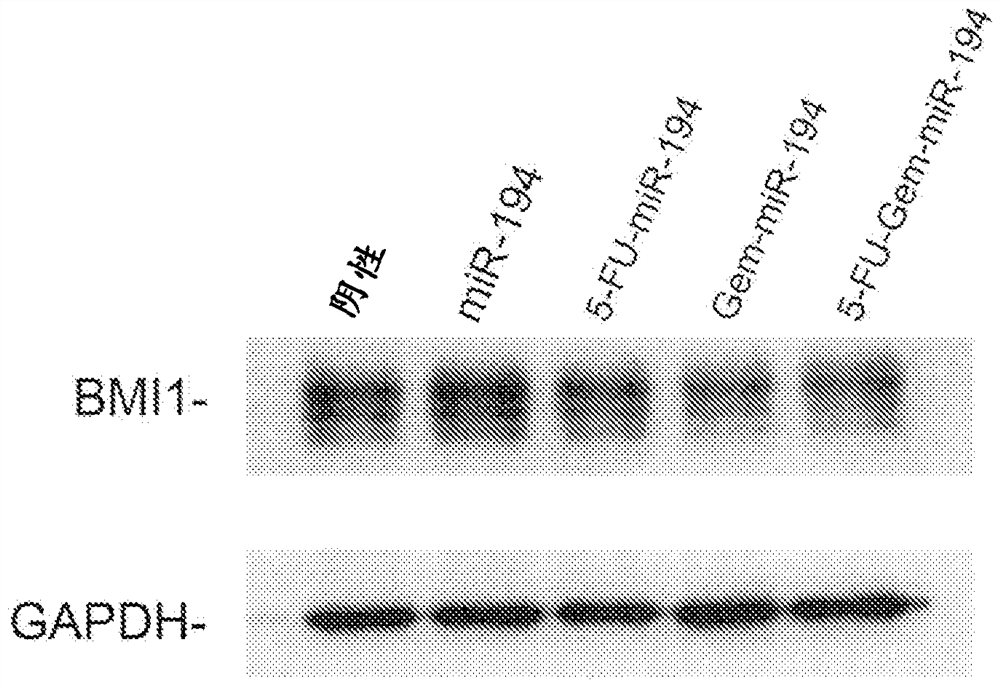
[0131] Target specificity analysis: The results of western blot experiments in pancreatic cells confirmed that exemplary modified miR-194 polynucleotides of the present disclosure were able to retain their target specificity for SET8 and BMI1. The results are displayed in Figures 2A ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com