Human EZH2 mutant gene and application thereof
A mutation gene and gene technology, applied in the field of genetic detection and application, can solve the problems of low ctDNA content, fragmentation, unsuitability, etc., and achieve the effect of rapid and accurate mutation detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] One case of gastric cancer patient (No. 1#) who was pathologically diagnosed in the hospital was selected. After signing the informed consent, two blood samples were collected during the treatment process, and gene capture and next-generation sequencing of ctDNA samples were performed. At the same time, The clinical treatment and condition evaluation information were collected, and finally a comprehensive analysis was performed (see Table 1 for details).
[0035] (1) Blood sample collection
[0036] Take 5ml of EDTA anticoagulant blood from the patient's cubital vein (see Table 1 for the time of blood collection), and separate plasma and monocytes within 1 hour. The plasma sample contains ctDNA, which can be used to reflect the information of the cancer focus, and the monocytes contain normal genomic DNA. for reference.
[0037] (2) Capture sequencing of EZH2 gene
[0038] The samples in (1) were sent to BGI Genomics on dry ice for gene capture and next-generation seq...
Embodiment 2
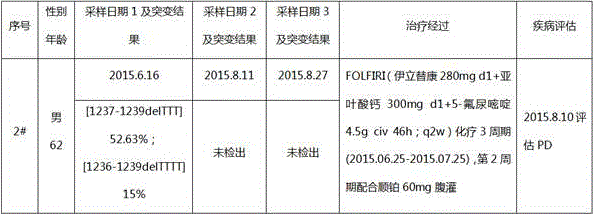
[0045] A patient with gastric cancer (No. 2#) who was pathologically diagnosed in the hospital was selected. After signing the informed consent, 3 blood samples were collected during the treatment process, and gene capture and next-generation sequencing of ctDNA samples were performed. At the same time, The clinical treatment and condition evaluation information were collected, and finally a comprehensive analysis was performed (see Table 2 for details). See embodiment 1 for the specific process.
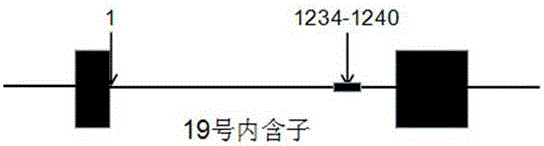
[0046] The patient's previous blood sample (2015.6.16) detected the deletion of three thymine nucleotides at the 1236-1238 position of intron 19 of the EZH2 gene, that is, the [1236-1238delTTT] mutation (9 times detected) , corresponding to intron 19 sequence as shown in SEQ ID NO:1, this mutation belongs to the mutation at the 1234-1240 position, and the mutation frequency is as high as 52.63%; in addition, this blood sample (2015.6.16) also detected The deletion of four thymine n...
Embodiment 3
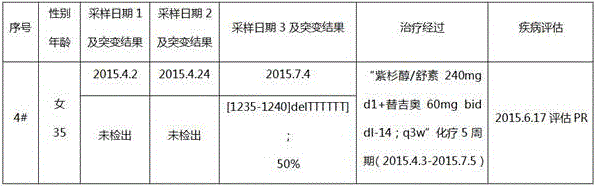
[0050] A patient with gastric cancer (No. 3#) who was pathologically diagnosed in the hospital was selected. After signing the informed consent, 3 blood samples were collected during the treatment process, and gene capture and next-generation sequencing of ctDNA samples were performed. At the same time, The clinical treatment and condition assessment information were collected, and finally a comprehensive analysis was performed (see Table 3 for details). See embodiment 1 for the specific process.
[0051] The patient's previous blood sample (2015.3.31) did not detect a mutation, but the middle blood sample (2015.5.15) detected the insertion of two thymine nucleotides at the 1234-1235 site of intron 19 of the EZH2 gene That is, [1234-1235insTT] mutation (detected twice), corresponding to the sequence of intron 19 as shown in SEQ ID NO: 5, this mutation belongs to the mutation at position 1234-1240, and the mutation frequency is as high as 50%; No mutation was detected in the l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com