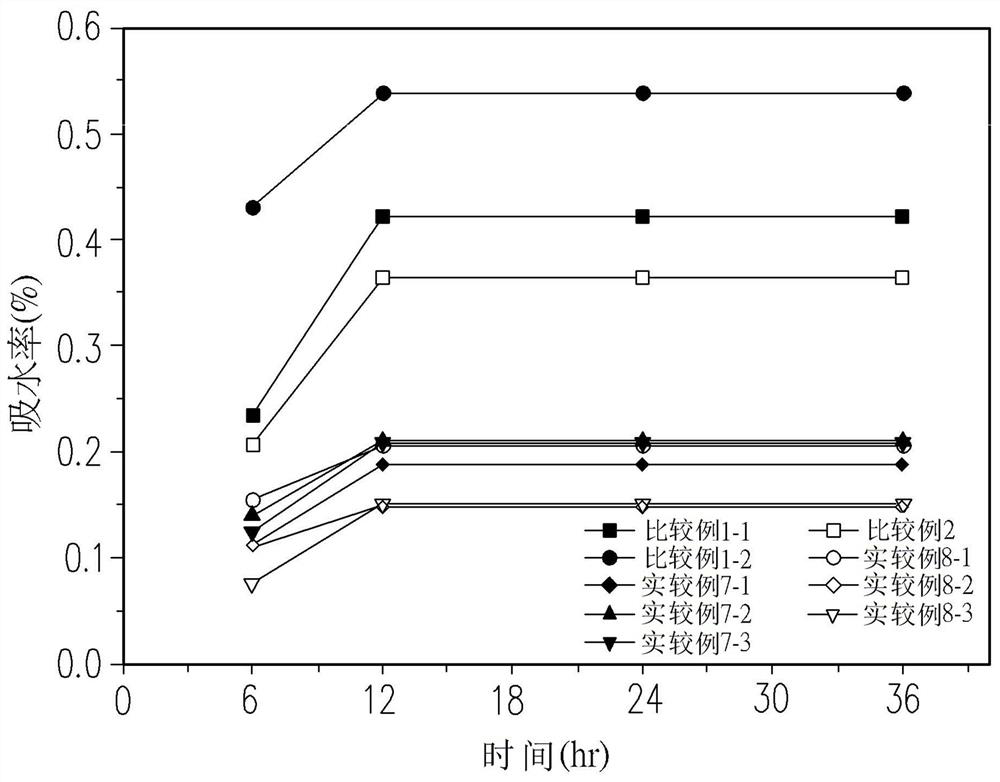
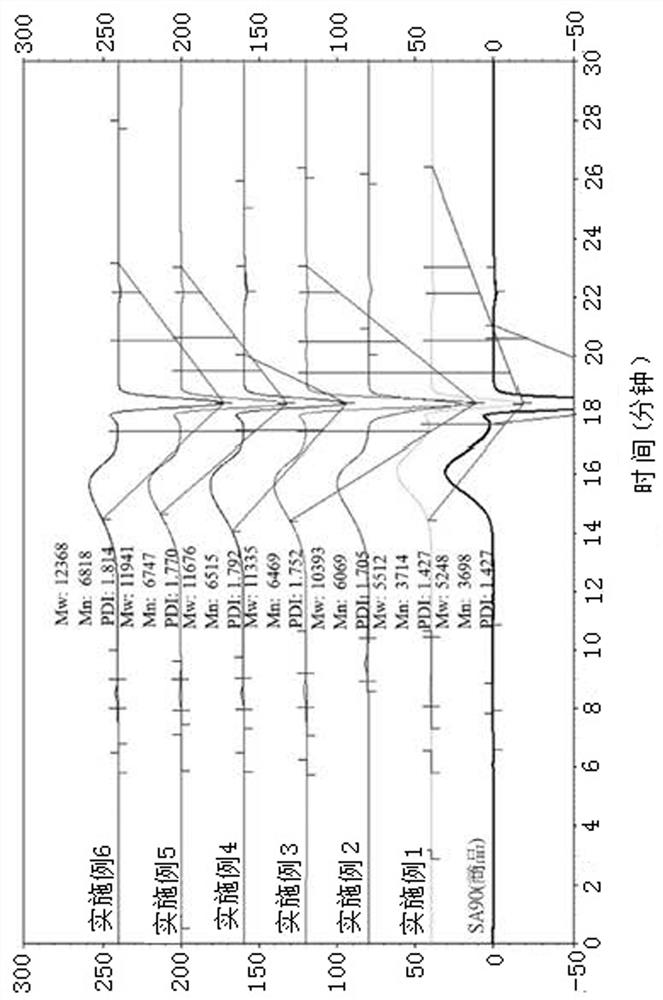
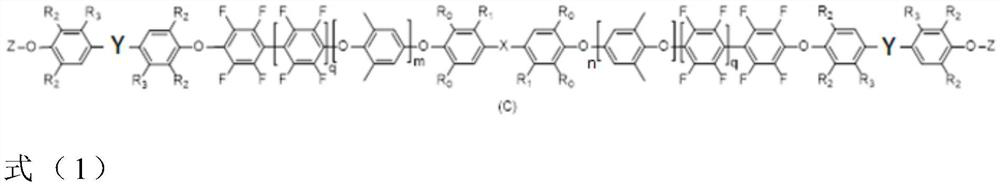
Oligomer (2,6-dimethylphenylene ether) Together with Fabrication Method and Cured Product Thereof
A technology of dimethylphenylene ether and oligomers, which is applied in the field of its preparation method and cured products and oligomers, which can solve the problems of reducing the force of intermolecular hydrogen bonds, difficult improvement of dielectric properties, and reduction of dielectric constant. , to achieve the effects of simplified steps, excellent organic solubility, and high glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053]
[0054] The preparation method of oligo(2,6-dimethylphenylene ether) in this embodiment includes the following steps.
[0055] First, step 1 is carried out, the (2,6-dimethylphenylene ether) oligomer at the end of the phenol shown in the formula (2) and the hexafluorobenzene ring or decafluorobiphenyl shown in the formula (3) are mixed in the base Under the catalysis of the active catalyst, the fluorine-containing (2,6-dimethylphenylene ether) oligomer represented by the formula (4) is obtained.
[0056]
[0057] In formula (2) - formula (4), R 0 with R 1 Each is independently hydrogen, C1-C6 alkyl or phenyl, X is -NR-, -CO-, -SO-, -CS-, -SO 2 -, -CH 2 -, -O-, null, -C(CH 3 ) 2 -or n and m are each independently an integer of 0 to 300. q is an integer of 0 to 1.
[0058] In one embodiment, the alkaline catalyst is potassium carbonate (K 2 CO 3 ), sodium carbonate (Na 2 CO 3 ), potassium hydroxide (KOH), sodium hydroxide (NaOH), sodium bicarbonate (NaH...
Embodiment 1
[0098] The synthesis of embodiment 1 fluorine-containing (2,6-dimethylphenylene ether) oligomer
[0099] In a 100 ml three-neck reactor, add 5 g (3.125 mmole) of SA90, 2.91 g (3.125*5 mmol) of Hexafluorobenzene (Hexafluorobenzene), 0.95 g (3.125*2.2 mmol) of potassium carbonate (K 2 CO 3 ) and 20 grams of dimethylacetamide (N,N-Dimethyl acetamide). Next, under a nitrogen atmosphere, the temperature was raised to 80° C., and the reaction was carried out for 36 hours. After the reaction, cool to room temperature. The mixture was poured into methanol water to precipitate, and washed several times with methanol water. Finally, suction and filtration, the filter cake was vacuum-dried at 60°C to obtain a white powdery product, as shown in the figure below, with a yield of 92%. Wherein, n and m are independently integers from 0 to 300.
[0100]
[0101] Next, the product's 1 H NMR spectrum. With high-resolution nuclear magnetic resonance spectrometer (400MHz Nuclear Magneti...
Embodiment 2
[0108] Example 2 Synthesis of fluorine-containing (2,6-dimethylphenylene ether) bisphenol oligomer
[0109]In a 100 ml three-neck reactor, add 2.34 g (1.553*4 mmole) of dicyclopentadiene-2,6-bisphenol, 0.47 g (3.125*5 mmol) of potassium carbonate and 4 g (1.553*2.2 mmol) of dimethyl Dimethylformamide was heated to 120° C. under nitrogen atmosphere and stirred for 30 minutes. Another 3 grams (1.553 mmole) of the fluorine-containing (2,6-dimethylphenylene ether) oligomer of Example 1 was dissolved in 18 grams of dimethylformamide, and then added dropwise to the three-neck reactor And maintained at 120°C for 12 hours. After the reaction, cool to room temperature. The mixture was poured into methanol water to precipitate, and washed several times with methanol water. Finally, air filtration was performed, and the filter cake was vacuum-dried at 60° C. to obtain a brown powder product, as shown in the figure below, with a yield of 85%. Wherein, n and m are independently integer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com