Therapeutic combinations of orally administered docetaxel and p-gp inhibitor for the treatment of cancer
A technology of docetaxel, cancer, applied in the field of compound A for treating cancer, compound for treating diseases or disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0855] Example 1 - Clinical study on the combination of multi-west race and compound A for oral administration with IV multi-West
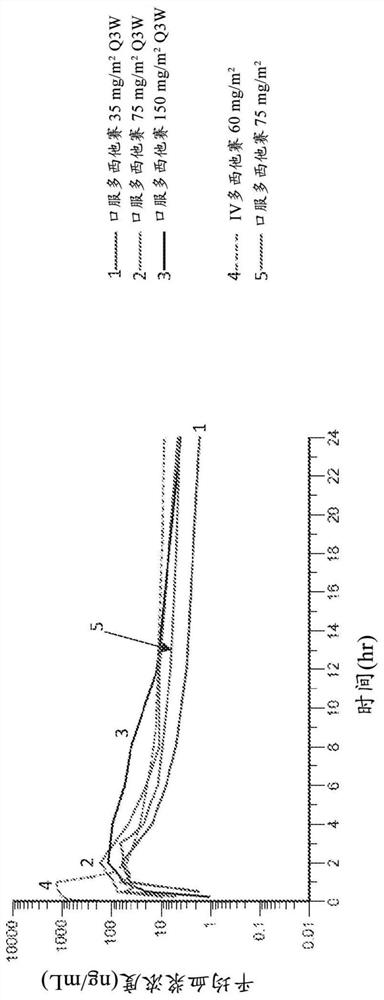
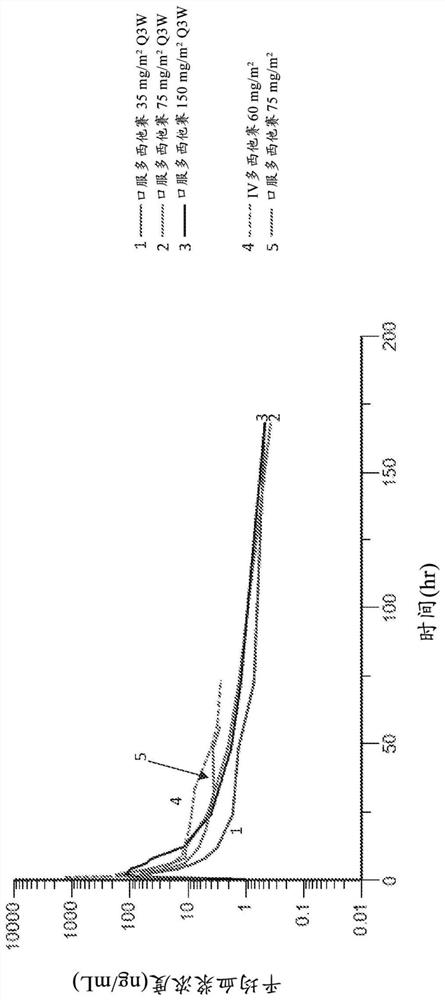
[0856] One study was carried out in a subject with metastatic prostate cancer ("study 1"). The initial stage includes a 21 days of IV application of Dozi Group (according to the dose specified for each participant, as the final treatment of the IV Dauxi His Tour). In the second part of this study, press 75mg / m 2 150mg / m 2 , Or 225mg / m 2 Start, as a single dose, oral dozo. A total of 5 (n = 18) Subjects participated in this study.
[0857] Compared to oral administration, an area is applied from 0 to 24 hours (AUC) 0-24 )higher. The plasma concentration in the multi-West, increases with the increase of IV administration (35, 75mg / m 2 . AUCs for the dose of IV and oral administration were 27.17 (Ng * HR / mL) and 23.33 (Ng * HR / mL), respectively. Half-life for oral dozi, T 1 / 2 It is 21.3h, compared to the half-life of the IV multi-West, is 21....
example 2
[0867] Example 2 - Clinical study on dose surveys of multi-Wests and Compound A combined with oral administration
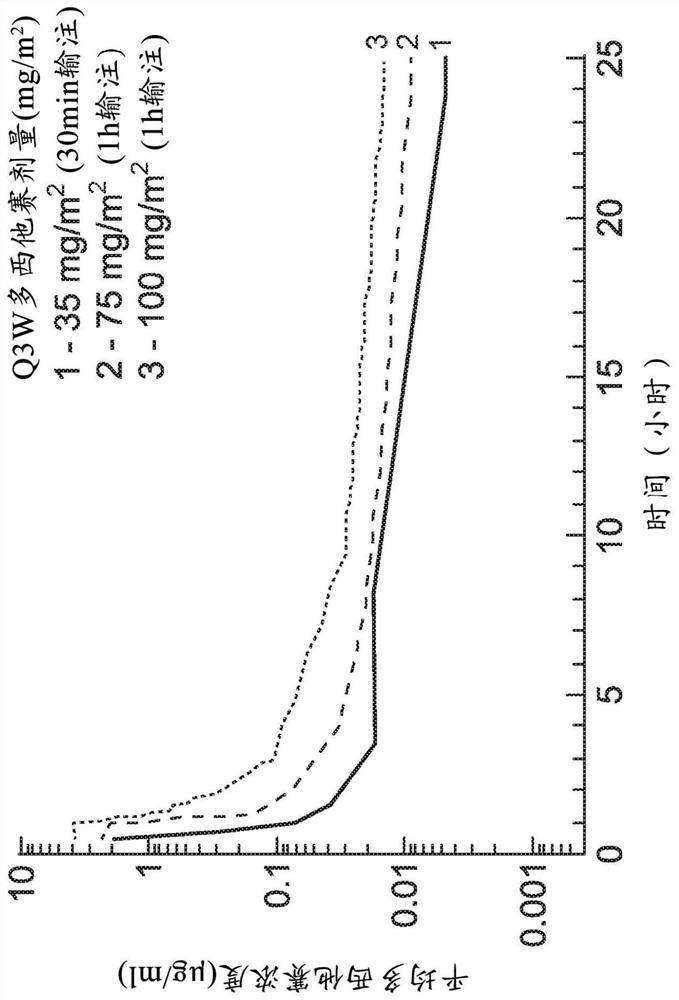
[0868] One study was carried out in a subject having a solid tumor ("study 2"). Press 35mg / m 2 The initial dose of the starting dose, the maximum tolerated dose (MTD) of Oral Dozi, including 21 days 3 + 3 dose increments. Dosage level is increased to 75 mg / m 2 150mg / m 2 225mg / m 2 300mg / m 2 375mg / m 2 , And 400mg / m 2 . In the second part of this study, a given group starting at half of the dose starting from stage 1 from stage 1 for two days in the second part of this study. A total of 15 (n = 40) subjects participated in this study.
[0869] Pharmacokinetic results from 2 studies showed 35 mg / m 2 75mg / m 2 , And 150mg / m 2 Dose maximum concentration C max The values were 69.1 ng / ml, 172 ng / ml, and 124 ng / ml. Under the 0 to 24-hour curve (AUC 0-24 As the dose increases, 263 ng * hr / ml is for 35 mg / m 2 Oral Dozi, 598 ng * hr / ml for 75mg / ...
example 3
[0876] Example 3 - Comparison of pharmacokinetic characteristics of multi-west race and compound A combined with oral administration (study 1 and research 2)
[0877] The plasma concentration of IV multi-West Herda increases with the increase of dose. Oral dozi he has a similar PK spectrum in two studies. The results are shown in Tables 6 and 7.
[0878] Table 6 Summary of PK parameters in the multi-West
[0879]
[0880] Table 7. Research 1 and Study 2 Title Comparison by Study on Cancer Diagnosis
[0881]
[0882]
[0883] In study, all subjects were diagnosed as prostate cancer. Oral dose is 75mg / m 2 Show C max In the range of 85.6-124 ng / ml, and AUC 0-24 For 313-336 ng * hr / ml. IV dose is 60mg / m 2 Show C max Within 1020-1570 ng / ml, and AUC 0-24 It is 1350-1970 ng * hr / ml.
[0884] In the study 2, cancer diagnosis includes lung cancer, breast cancer, esophageal cancer, lung cancer, urinary tractocarcinoma, pancreatic cancer, cervical cancer, ovarian cancer, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com