Medicament for treating cancer
A drug and prodrug technology, applied in the field of drugs for the treatment of hepatocellular carcinoma, can solve the problems of limited therapeutic effect, unconfirmed effect, low effective rate, etc., and achieve the effect of lasting anti-tumor effect and strong anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] (i) Antigen preparation
[0074] Information on the amino acid sequence (SEQ ID NO: 2) of hDLK-1 is published, for example, on the website of NCBI (GenBank) (http: / / www.ncbi.nlm.nih.gov / ) as "Accession Number: NP_003827". Also, information on the base sequence (SEQ ID NO: 1) encoding the amino acid sequence of hDLK-1 is published on the same website as "Accession No.: NM_003836".
[0075] As the antigen, a polypeptide or peptide (also simply referred to as a peptide) containing at least a part (all or part) of the amino acid sequence of hDLK-1 can be used, preferably an amino acid sequence containing the extracellular domain (FA-1) of hDLK-1 at least a part (all or part) of the peptide. The extracellular region of hDLK-1 is a region containing six EGF-like motifs (EGF-1 to EGF-6) as described above, and contains the 24th to 244th positions in the amino acid sequence shown in SEQ ID NO: 2 The amino acid region is preferably a region consisting of amino acids from "24th...
Embodiment 1
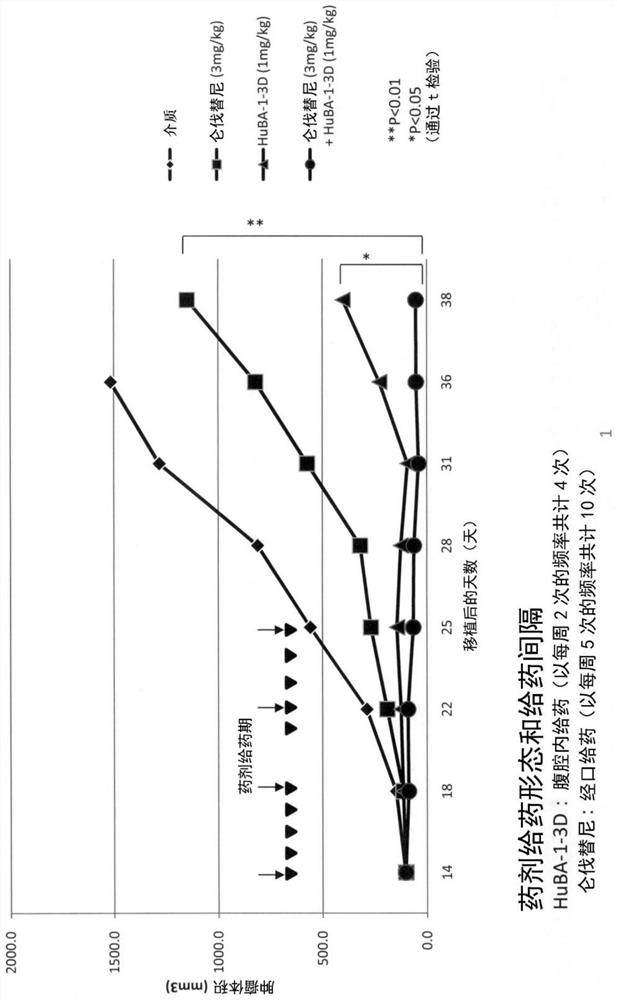
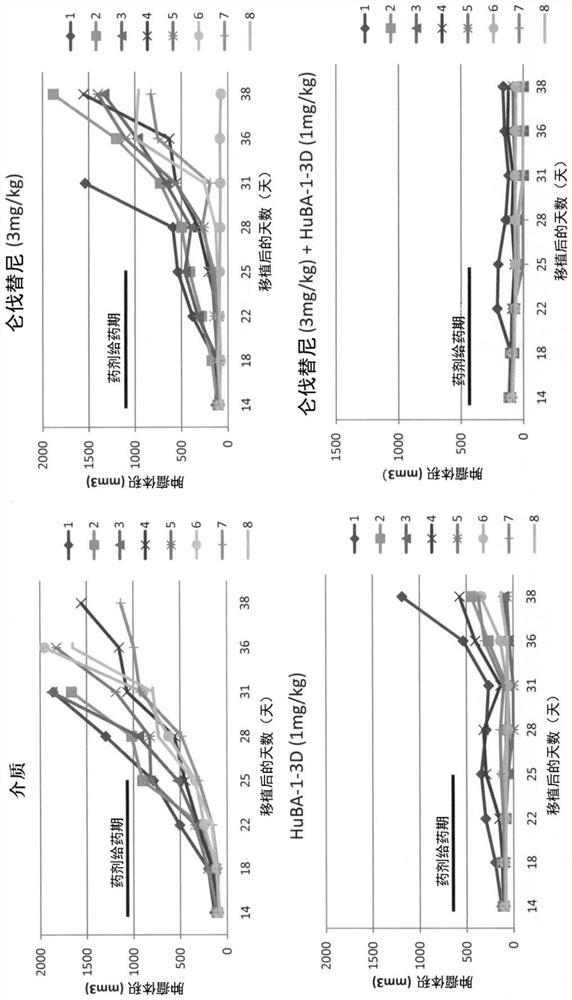
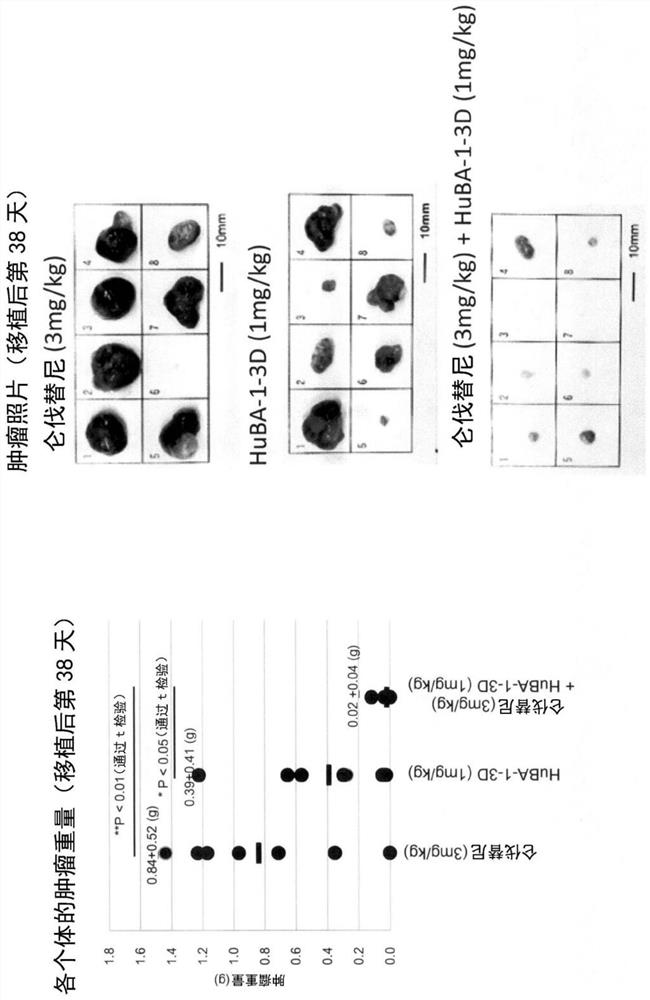
[0184] Using the Hep3B xenograft tumor model, the combined effect of lenvatinib mesylate and anti-hDLK-1 antibody in tumor formation inhibitory activity was investigated.
[0185] Here, a commercially available lenvatinib mesylate was used (hereinafter, simply referred to as "lenvatinib" in this Example.). The anti-hDLK-1 antibody used in this example is the "HuBA-1-3D-1-A24G / T73K antibody" described in WO2014 / 054820 (hereinafter referred to as "HuBA-1-3D antibody" in this example for short). ), the DNA encoding the antibody protein was constructed according to the method described in the same publication (Example in the specification), and then, for the production / production of the antibody protein using host cells, GlymaxX (registered trademark) technology was used (cf. ProBioGen AG; https: / / www.probiogen.de / genetic-glyco-engineering-adcc-glymaxx.html). It should be noted that the HuBA-1-3D-1-A24G / T73K antibody used in this example is composed of the amino acids shown in SE...
Embodiment 2
[0191] Using the HepG2 xenograft tumor model, the combined effect of lenvatinib mesylate and anti-hDLK-1 antibody in tumor formation inhibitory activity was investigated.
[0192] Here, a commercially available lenvatinib mesylate was used (hereinafter, simply referred to as "lenvatinib" in this Example.). The anti-hDLK-1 antibody used in this example is the "HuBA-1-3D-1-A24G / T73K antibody" described in WO2014 / 054820 (hereinafter referred to as "HuBA-1-3D antibody" in this example for short). ), the DNA encoding the antibody protein was constructed according to the method described in the same publication (Example in the specification), and then, for the production / production of the antibody protein using host cells, GlymaxX (registered trademark) technology was used (cf. ProBioGen AG; https: / / www.probiogen.de / genetic-glyco-engineering-adcc-glymaxx.html). It should be noted that the HuBA-1-3D-1-A24G / T73K antibody used in this example is composed of the amino acids shown in SE...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com