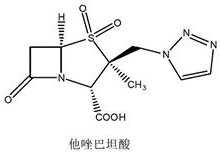
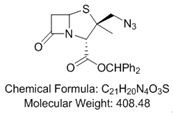
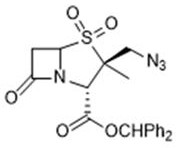
Preparation method of 2β-azidomethylpenicillanic acid diphenylmethyl ester, tazobactam intermediate and tazobactam
A technology of diphenylmethyl azidomethylpenicillanic acid and tazobactam, which is applied in the field of preparation of medicines, can solve the problems of high cost and low yield, and achieve increased yield, improved conversion rate, shortened The effect of the reaction step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] In a typical implementation of the present application, a preparation method of 2β-azidomethylpenicillanic acid diphenylmethyl ester is provided, the preparation method comprising: comprising a disulfide ring-opening compound, an azide source , The reaction raw material of oxidizing agent carries out free radical reaction in solvent, obtains the product system that comprises 2β-azidomethyl penicillanic acid diphenylmethyl ester, wherein, the structural formula of disulfide ring-opening compound is , the oxidizing agent causes the azide source to generate azide radicals.
[0029] The oxidant causes the azide source to generate azide radicals, and the carbon-carbon double bond of the disulfide ring-opening compound undergoes free radical addition through the azide radical, and then intramolecular free radical substitution successfully realizes the direct conversion of the disulfide ring-opening compound to Efficient and highly selective synthesis of benzhydryl 2β-azidome...
Embodiment 1
[0052]
[0053] Dissolve disulfide compound 2 (10 g, 18.77 mmol) in THF (70 mL) at room temperature and stir to clarify, then add PhI(OAc) 2 solid (4.84 g, 15.02 mmol, 0.8 eq), the system was cooled to 5 °C, and then slowly added TMS-N 3 (2.38 g, 20.65 mmol, 1.1 eq) and tetrahydrofuran (30 mL) mixed solution, the temperature of the process control system was 5 ℃. After dropping, the system was kept at this temperature and stirred for 6 hours. After the reaction of the raw materials was completed, the system was reddish-brown turbid. After filtering to remove the bithiazole by-product, the filtrate was quenched with 10wt% sodium thiosulfate aqueous solution, and dichloromethane (50 mL) was added. Separation, organic phase detection HPLC external standard, system external standard yield 85%, intermediate 4 (2β-azidomethyl penicillanic acid diphenylmethyl ester) product HPLC purity 95%, temperature control 30~40 °C, concentrated under reduced pressure until no distillate was ...
Embodiment 2
[0066] Dissolve disulfide ring-opening compound 2 (10 g, 18.77 mmol) in acetonitrile (150 mL) and stir to clarify at room temperature, then add 10 mL of sodium azide aqueous solution (2.24 g, 37.54 mmol, 2 eq) and 10 mL of zinc chloride Aqueous solution (512 mg, 3.75 mmol, 0.2 eq), the system was cooled to 0 °C, stirred for 30 min, and then a mixed solution of ceric ammonium nitrate (20.58 g, 37.54 mmol, 2 eq) and water (30 mL) was slowly added dropwise to the system, The temperature of the process control system was 0 °C, and there were gassing and exothermic phenomena during the reaction. After dripping, the system was kept at this temperature and stirred for 60 minutes. The reaction of the raw materials was completed. The system was red and turbid. After filtering to remove the bithiazole by-product, the filtrate was quenched with 10% aqueous sodium thiosulfate solution, and dichloromethane (50 mL) was added to carry out the reaction. Separation, organic phase detection HPL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com