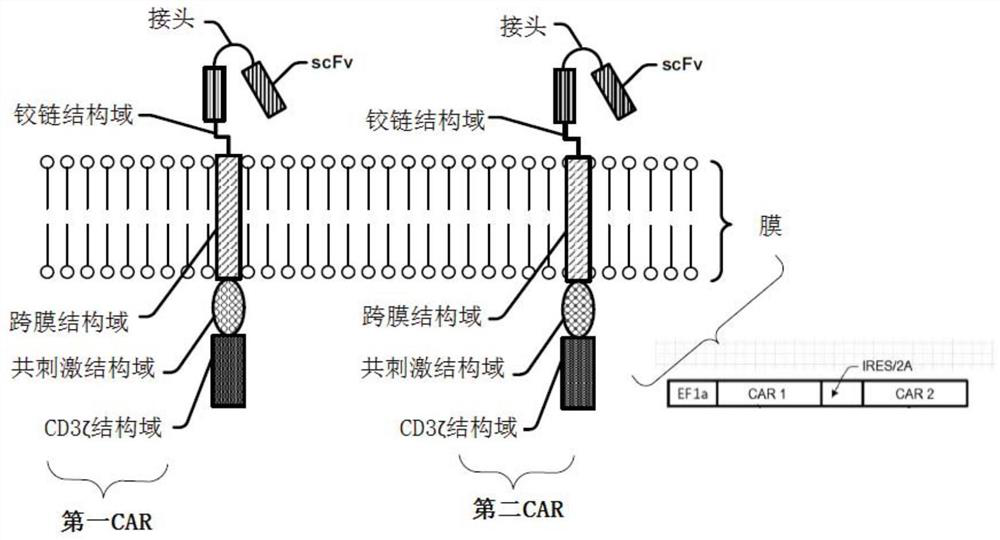
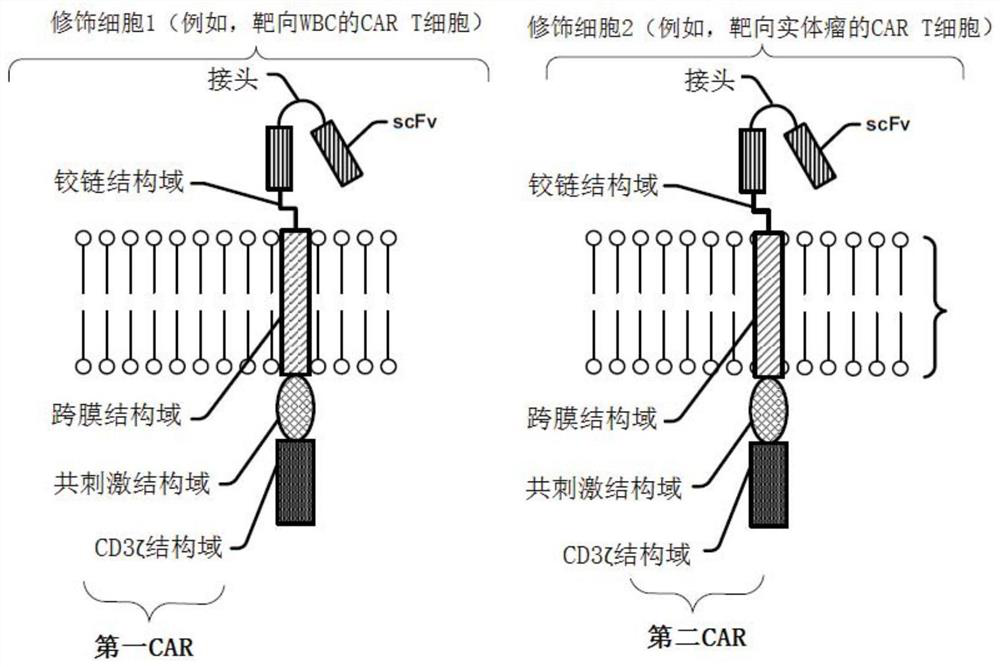
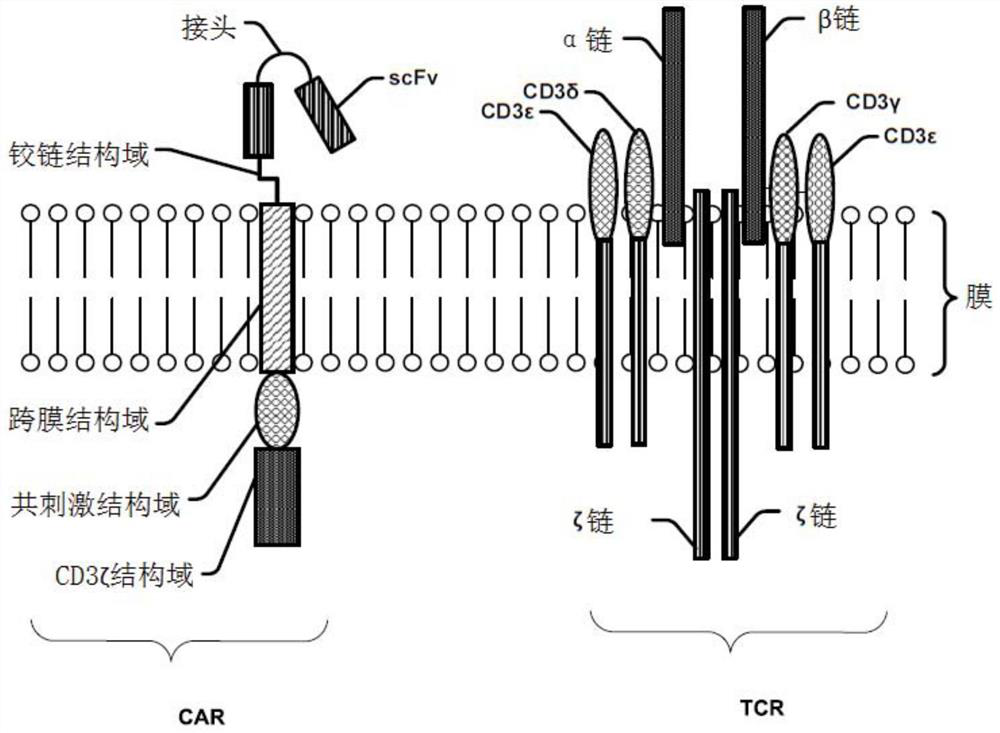
Modified cell expansion and uses thereof
A technology of cells and cell groups, which is applied in the field of expansion of modified cells and its application, and can solve the problems of slow progress in the treatment of solid tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0474] Example 1. Bispecific CAR
[0475] Lentiviral vectors encoding the individual CAR molecules were generated and transfected with T cells as detailed below. For technologies related to cell culture and cytotoxic T lymphocyte assay construction, please refer to "Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains", PNAS, March 3, 2009, Volume 106, Issue 9 , pp. 3360-3365 and "Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of TCells and Increased Antileukemic Efficacy In Vivo," Molecular Therapy, 2009, Vol. 17 No. 8, pp. 1453-1464, cited by reference method is incorporated into this article as a whole.
[0476] On day 0, peripheral blood was drawn from healthy volunteers and sorted to collect CD3+ T cells. CD3 / CD28 Dynabeads were added to collected CD3+ T cells at a ratio of 1:1. On day 1, a vector comprising CD19 CAR (MOI=15; the binding domain of CAR is SEQ...
Embodiment 2
[0503] Example 2. CAR T cell expansion and antitumor activity in patients
[0504] The clinical study was designed to assess the safety and efficacy of infusion into patients of autologous T cells modified to express CAR / 4-1BB / CD3-ζ specific for several solid tumor markers. In the first arm (arm) of the study, patients received only solid tumor marker-specific CAR T cells. Solid tumor markers include TSHR and tMUC1. In the second cohort, patients received CAR T cells directed against CD19 and solid tumor antigens (eg, TSHR, tMUC1, or GUCY2C). T cells from the patient are obtained, modified and infused into the patient. T cell responses of patients from the first and second groups were measured and compared using the following protocol approved by the hospital conducting the trial. Written informed consent was provided to all patients. Information on these patients is provided in Table 9 below (SD: stable disease; PD: progressive disease; PR: partial response; CR: complete ...
Embodiment 3
[0533] Example 3. Activation of coupled / mixed T cells
[0534] Mixed CAR T cells (coupled CAR T cells) were divided into three groups for activation assays: CD19 CAR and tMUC1 CAR (group 1), anti-CD19 CAR and ACPP CAR (group 2), and CD19 and CLDN18.2 CAR (group 2). Group 3). Peripheral blood was collected from healthy volunteers. CD3+ T cells were sorted with Pan T kit and added to CD3 / CD28 Dynabead at a ratio of 1:1. CD3+ T cells were then transfected with lentivirus. Remove lentivirus and Dynabead and add fresh medium. Determine CAR ratio and cell phenotype. CAR expression in these three groups of cells was measured. CD19 CAR T cells, tMUC1 CAR T cells and target cells were selected and mixed for 24 hours or 48 hours. The expression of various markers in the corresponding cells was measured. Will 20x10 4 CAR T cells and 20x 10 4 Substrate cells were co-cultured for 24 hours. The expression of molecules such as hCAR (humanized scFv), mCAR (murine scFv), CD25 and CD1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com