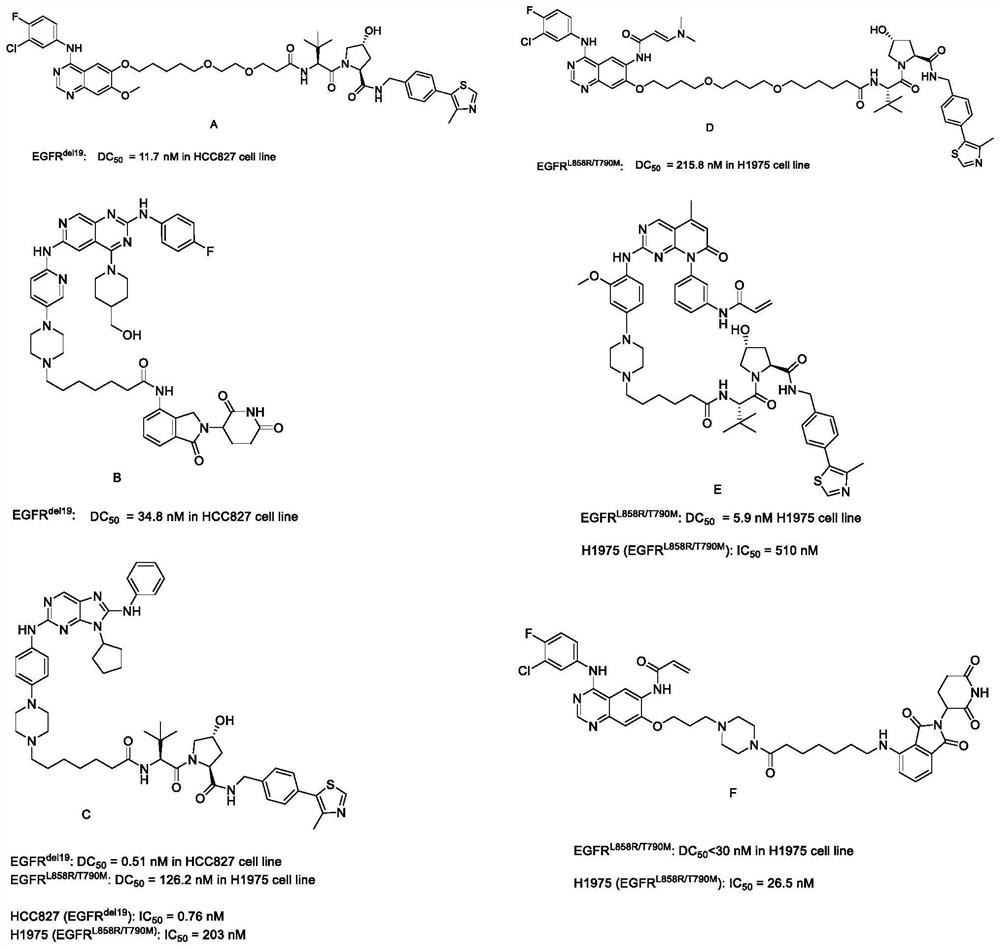
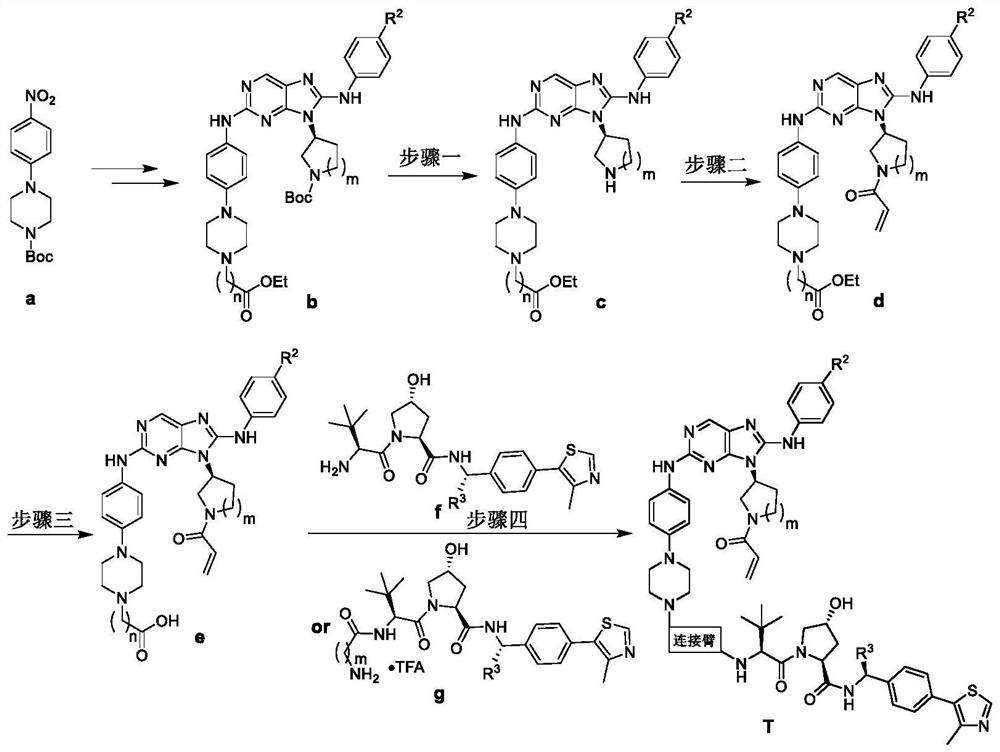
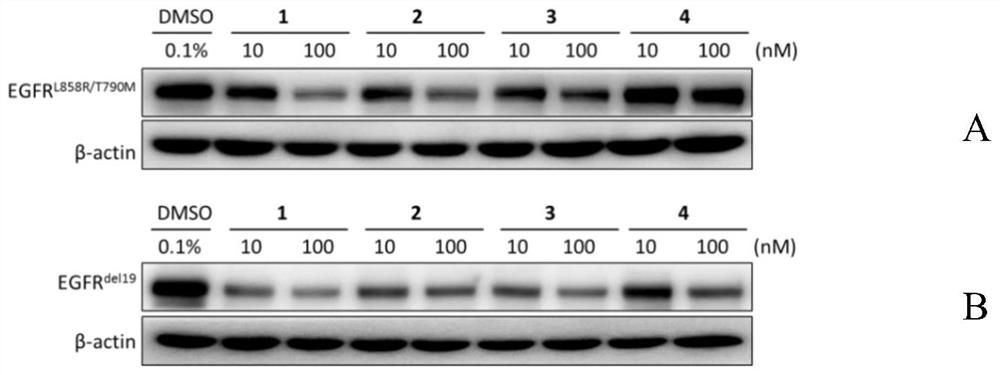
EGFR degradation agent containing 2, 8, 9-trisubstituted-9H-purine structure fragment and salt and application of EGFR degradation agent
A technology of structural fragments and three substitutions, applied in the field of anticancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: (2S,4R)-1-((S)-2-(7-(4-(4-((9-((S)-1-acryloyl-3-pyrrolidinyl))-8 -anilino-9H-2-purinyl)amino)phenyl)-1-piperazinyl)heptanylamino)-3-(3,3-dimethyl)-butyryl)-4-hydroxyl-N- Synthesis of (4-(4-methyl-5-thiazolyl)benzyl)pyrrolidine-2-carboxamide (structural formula 1)
[0039] Step 1: (S)-2-((4-(4-(7-ethoxy-7-oxoheptyl)-1-piperazinyl)phenyl)amino)-8-phenylamino-9-( Synthesis of 3-pyrrolidinyl)-9H-purine (c1)
[0040]
[0041] References J.Med.Chem.2012,55,10685-10699, Eur.J.Med.Chem.2020,186,111888 and Eur.J.Med.Chem.2020,208,112781 synthetic intermediate b1; reference Eur.J.Med.Chem.2020,186,111888 prepared c1 with a yield of 60%.
[0042] Step 2: (S)-2-((4-(4-(7-ethoxy-7-oxoheptyl)-1-piperazinyl)phenyl)amino)-8-phenylamino-9-( Synthesis of N-acryloyl-3-pyrrolidinyl)-9H-purine (d1)
[0043]
[0044] Suspend c1 (0.27g, 0.57mmol) in acetone (2mL), cool and stir in an ice bath for 5min, then dissolve acryloyl chloride (70μL, 0.86mmol) in acetone (3mL) and...
Embodiment 2
[0051] Example 2: (2S,4R)-1-((S)-2-(7-(4-(4-((9-((S)-1-acryloyl-3-piperidinyl)-8) -anilino-9H-2-purinyl)amino)phenyl)-1-piperazinyl)heptanylamino)-(3,3-dimethyl)butyryl)-4-hydroxy-N-(4- Synthesis of (4-methyl-5-thiazolyl)benzyl)pyrrolidine-2-carboxamide (structural formula 2)
[0052] The preparation of compound 2 was the same as in Example 1. HRMS(ESI): Calculated value: 1064.56566[M+H] + , measured value: 1064.57031.
Embodiment 3
[0053] Example 3: (2S,4R)-1-((S)-2-(8-(4-(4-((9-((S)-1-acryloyl-3-piperidinyl)-8 -anilino-9H-2-purinyl)amino)phenyl)-1-piperazinyl)octanoylamino)-(3,3-dimethyl)butyryl)-4-hydroxy-N-(4- Synthesis of (4-methyl-5-thiazolyl)benzyl)pyrrolidine-2-carboxamide (structural formula 3)
[0054] The preparation of compound 3 was the same as in Example 1. HRMS(ESI): Calculated value: 1078.58131[M+H] + , measured value: 1078.58234.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com