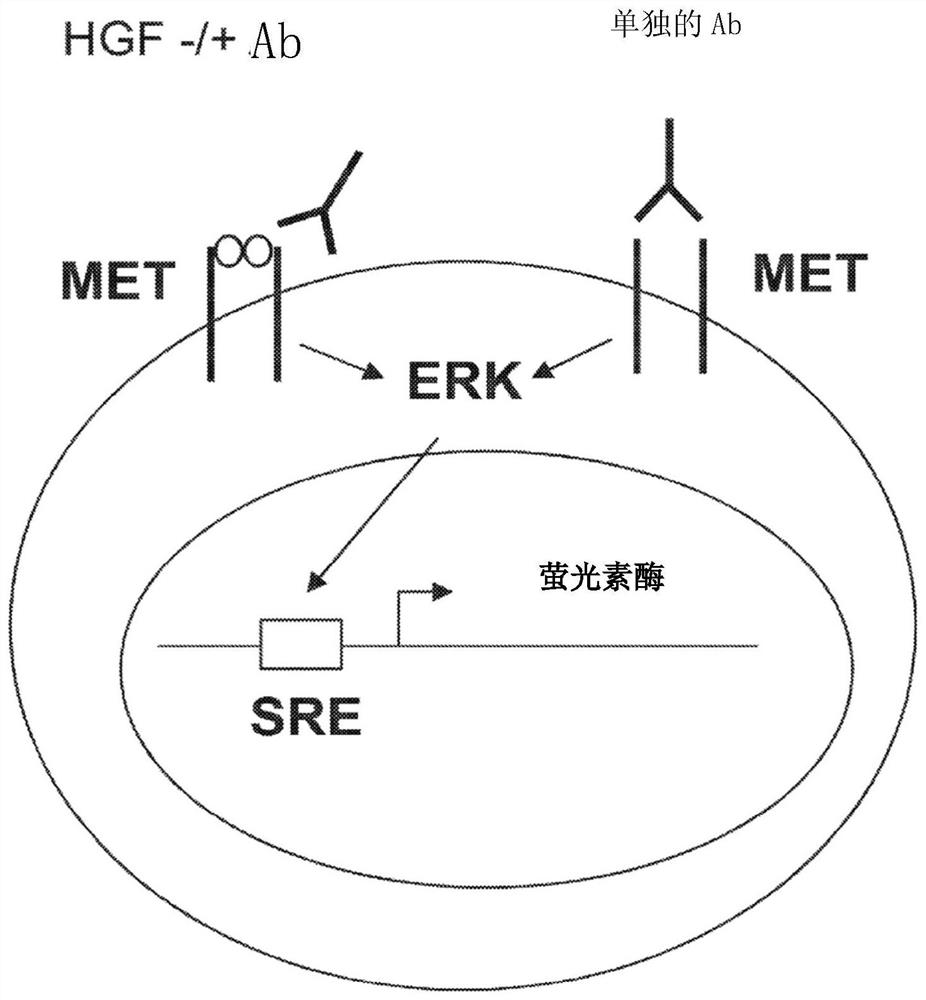
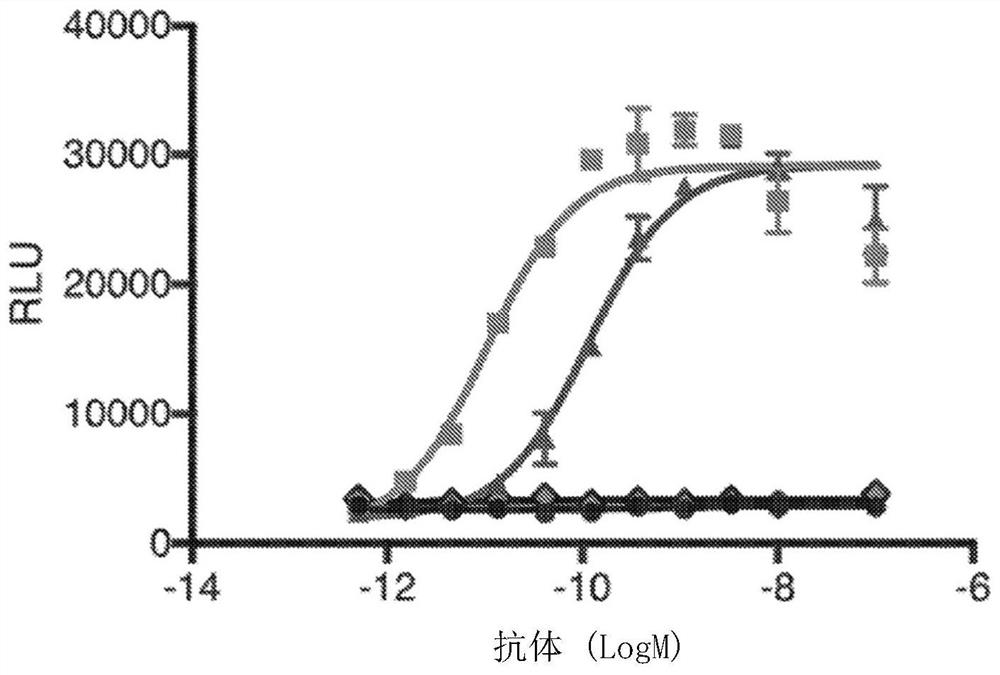
Methods of treating ocular cancer using Anti-met antibodies and bispecific antigen binding molecules that bind met
An antigen-binding molecule and bispecific technology, applied in anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, chemical instruments and methods, antibodies, etc., can solve problems such as unmet medical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0407] Production of Human Antibodies
[0408] Anti-MET antibodies and MET x MET bispecific antibodies useful according to the methods provided herein can be fully human antibodies. Methods for producing monoclonal antibodies, including fully human monoclonal antibodies, are known in the art. Human antibodies that specifically bind human MET can be prepared using any such known method in the context of the present disclosure.
[0409] For example, high affinity chimeric antibodies having human variable regions and the MET of mouse constant regions are initially isolated using VELOCIMMUNET™ technology or any other similar known method for producing fully human monoclonal antibodies. Antibodies are characterized and selected for desirable characteristics, including affinity, ligand blocking activity, selectivity, epitope, etc., as in the experimental section below. If necessary, mouse constant regions are replaced with desired human constant regions, such as wild-type or modif...
Embodiment 1
[0447] Example 1. Production of Anti-MET Antibodies
[0448] Cells comprising DNA encoding human immunoglobulin heavy chain and kappa light chain variable regions were immunized with an immunogen comprising a recombinant human MET extracellular domain fused to a human Fc (R&D Systems, catalog number 358-MT, Minneapolis, MN). Genetically engineered mice to obtain anti-MET antibodies. The mice used for immunization express the "universal light chain". That is, antibodies produced in this mouse have different heavy chain variable regions, but substantially identical light chain variable domains.
[0449] Antibody immune responses were monitored by MET-specific immunoassays. When the desired immune response is achieved, splenocytes are harvested and fused with mouse myeloma cells to maintain their viability and form hybridoma cell lines. Hybridoma cell lines were screened and selected to identify cell lines producing MET-specific antibodies. Using this technique, several anti-...
Embodiment 2
[0451] Example 2. Heavy and light chain variable region amino acid and nucleic acid sequences
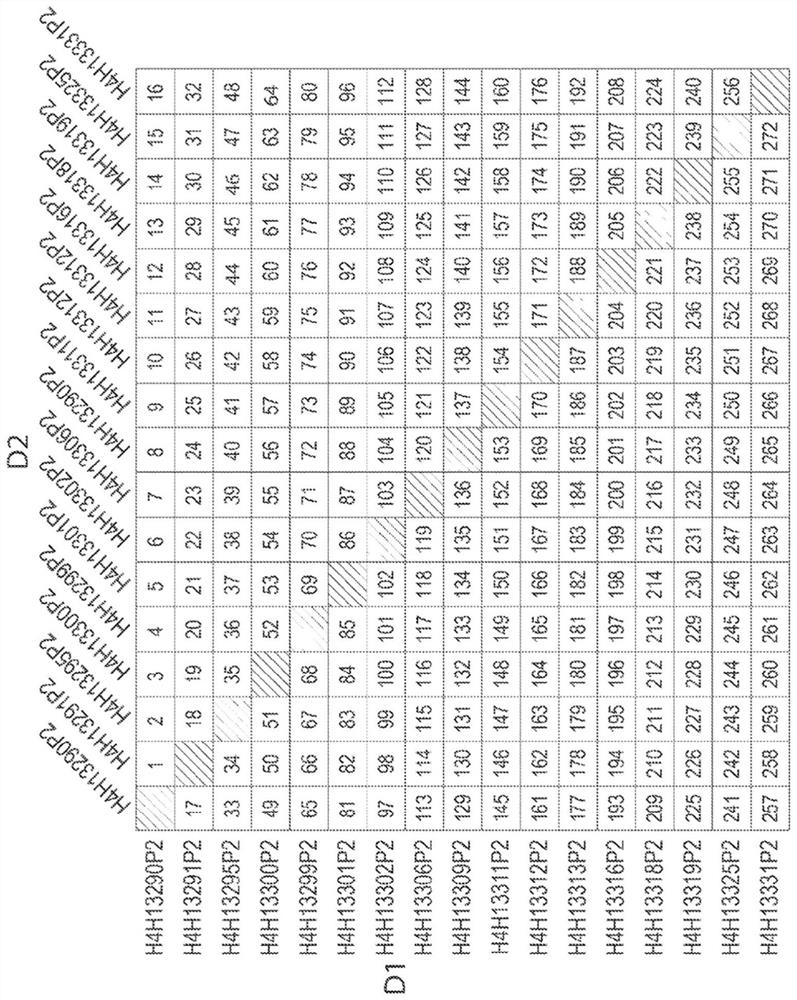
[0452] Table 1 lists the amino acid sequence identifiers for the heavy and light chain variable regions and CDRs of selected anti-MET antibodies described herein. (As noted above, all antibodies generated in Example 1 had identical light chain variable regions, and thus also identical light chain CDR sequences). The corresponding nucleic acid sequence identifiers are listed in Table 2.
[0453] Table 1: Amino Acid Sequence Identifiers
[0454]
[0455]
[0456] Table 2: Nucleic acid sequence identifiers
[0457]
[0458]
[0459] Antibodies are generally referred to herein according to the following nomenclature: an Fc prefix (e.g., "H4H"), followed by a numerical identifier (e.g., "13290," "13291," "13295," etc.), followed by a "P2" suffix, such as Tables 1 and 2 are shown. Accordingly, according to this nomenclature, antibodies may be referred to herein, eg, "H4H13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com