Construction method and application of glioblastoma organ model
A technology of glioblastoma and organoids, which is applied in the field of construction of glioblastoma organoid models, which can solve the problems of invasion and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the culture of cerebral cortex organoid
[0034] Refer to "Generation of cerebral organoids from human pluripotent stemcells" (Lancaster MA, Knoblich JA. Nature protocols 2014; 9:2329-2340.) to culture cerebral cortical organoids.
[0035] Human induced pluripotent stem cells were purchased from System Biosciences (product number: SC101A-1). The culture process is as follows: human induced pluripotent stem cells were recovered and cultured, and the feeder layer cells were radiation-inactivated mouse fibroblasts, which were purchased from MTI-GlobalStem. First, resuscitate the human induced pluripotent stem cells in the liquid nitrogen tank, preheat the fresh medium in a 37°C constant temperature water tank in advance, and prepare sterile straws / centrifuge tubes / culture bottles. After the fresh medium returns to temperature, spray 70% alcohol and wipe it clean, and immediately move it into the sterile operating table. The principle of resuscitating cells ...
Embodiment 2
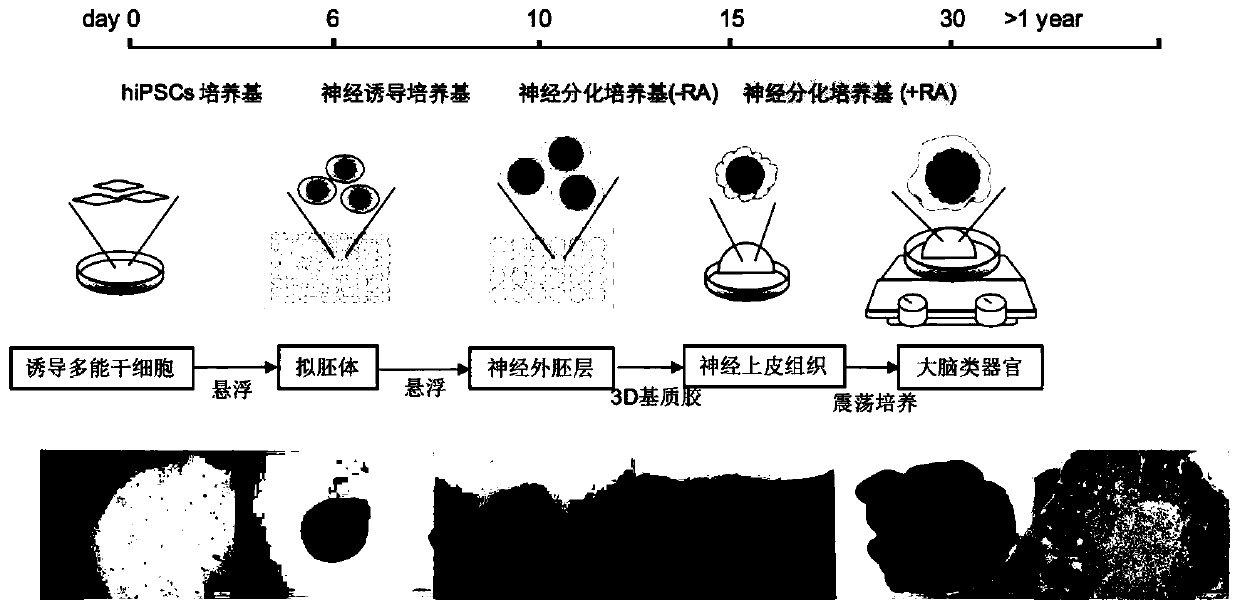
[0037] Example 2. Morphological Observation of Cerebral Cortical Organoids at Different Developmental Stages During Culture
[0038] Cerebral cortical organoids have different characteristics in each differentiation process, and need to be observed regularly and ready for the next step at any time. Human induced pluripotent stem cells (hiPSCs) in the logarithmic growth phase were seeded on culture dishes pretreated with irradiation-inactivated mouse fibroblasts (iMEFs), and hiPSCs spontaneously formed after 6 days of low-adhesion suspension culture in whole stem cell medium Cell clusters of uniform size, that is, embryoid bodies. At this time, the tissue has three layers of germ layers: inner, middle, and outer; use neural induction medium to make it oriented to differentiate into neuroectoderm, and gradually differentiate into translucent on the 10th day. A round cell mass with a darker center and clear and smooth edges, indicating the formation of neuroectoderm; cultured in ...
Embodiment 3
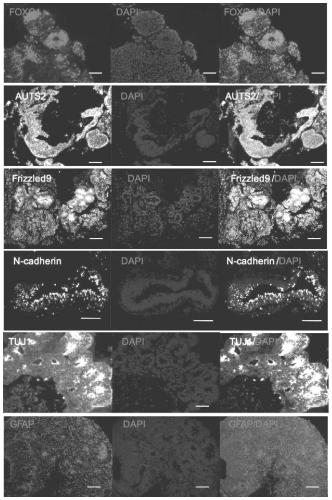
[0040] Example 3. Staining and Identification of Cultured Cerebral Cortical Organoids
[0041] After tissue fixation, dehydration, clearing, paraffin immersion and paraffin embedding, the cerebral cortical organoids were sectioned for H&E staining, and the expression of different marker proteins in the cerebral cortical organoids was detected by immunofluorescence. The formation of cerebral cortical organoids was confirmed by detecting the marker proteins of different brain regions and nerve cells on the cerebral cortical organoids cultured for 30 days. Marker proteins include forebrain marker protein FOXG1 / EMX1, hippocampus marker protein Frizzled9 / Isl1, midbrain marker protein Otx2, cerebellum or hindbrain marker protein En2 / Islet-1 / Nell2, neural stem cell marker protein N-cadherin, forehead Cortical marker protein Auts2, neuron and cortical development marker protein Pax6, glial cell marker protein GFAP and nerve cell marker protein TUJ1, etc. See the results figure 2 . ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com