Drug management method and system in trial drug clinical test process
A technology of clinical trials and management methods, applied in the field of drug management, can solve problems such as low drug deployment efficiency, achieve the effects of improving drug deployment efficiency, preventing inaccurate data, and preventing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
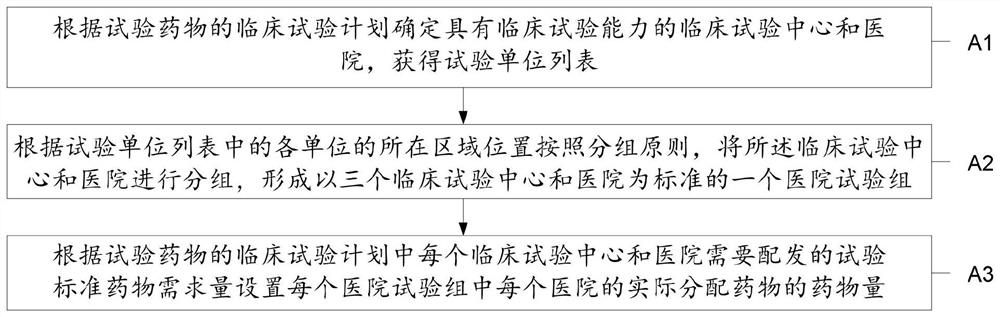
[0072] The preferred embodiments of the present invention will be described below in conjunction with the accompanying drawings. It should be understood that the preferred embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
[0073] The present invention proposes a drug management method in the trial drug clinical trial process, such as figure 1 As shown, the drug management method includes:
[0074] S1. According to the clinical trial plan of the trial drug, obtain the number of people assigned to each clinical trial center and hospital and the actual distribution of the corresponding trial drug, and distribute the actual distribution of the clinical trial center and hospital, the number of people in the trial, and the corresponding trial drug Quantities are integrated into a clinical trial checklist;
[0075] S2. Send the clinical trial list to the drug delivery department, and the drug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com