Product for predicting sensitivity of melanoma patient to MAGE-A3 immunotherapy
A MAGE-A3, melanoma technology, applied in the field of biomedicine, can solve problems such as unpredictability, and achieve the effect of high accuracy and good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
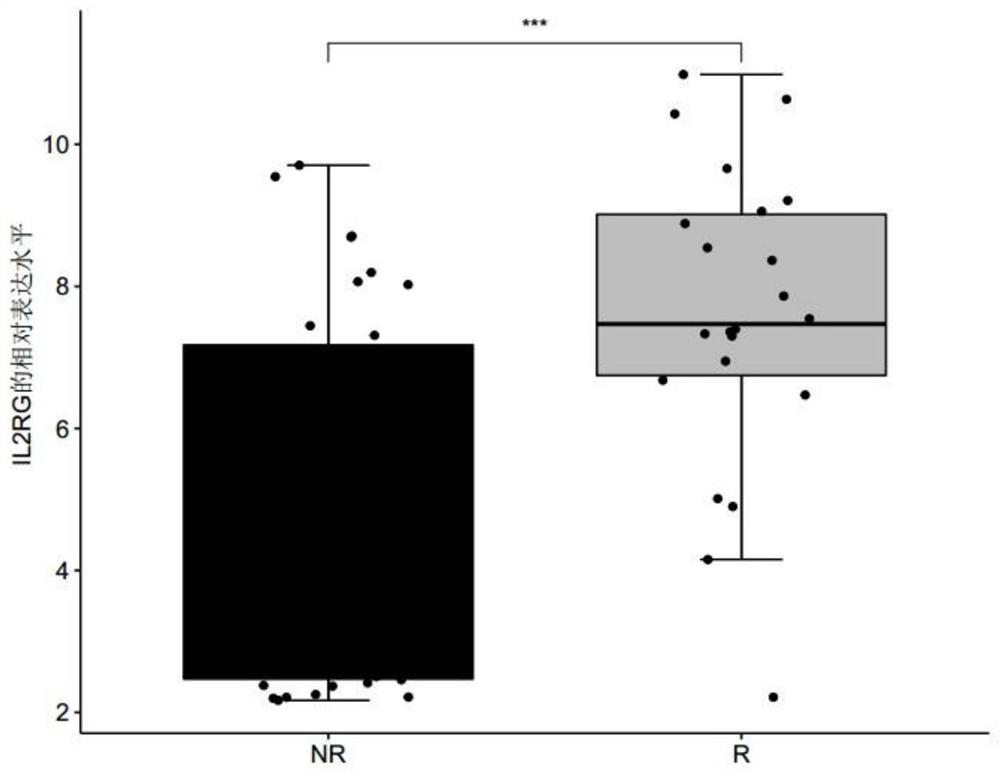
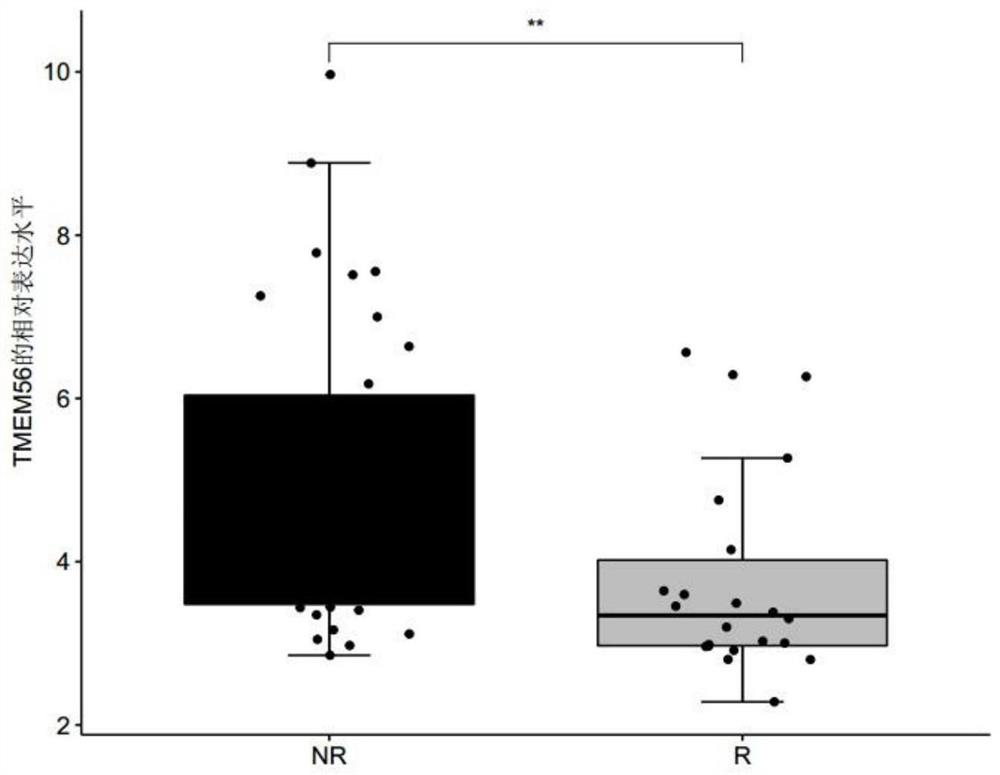
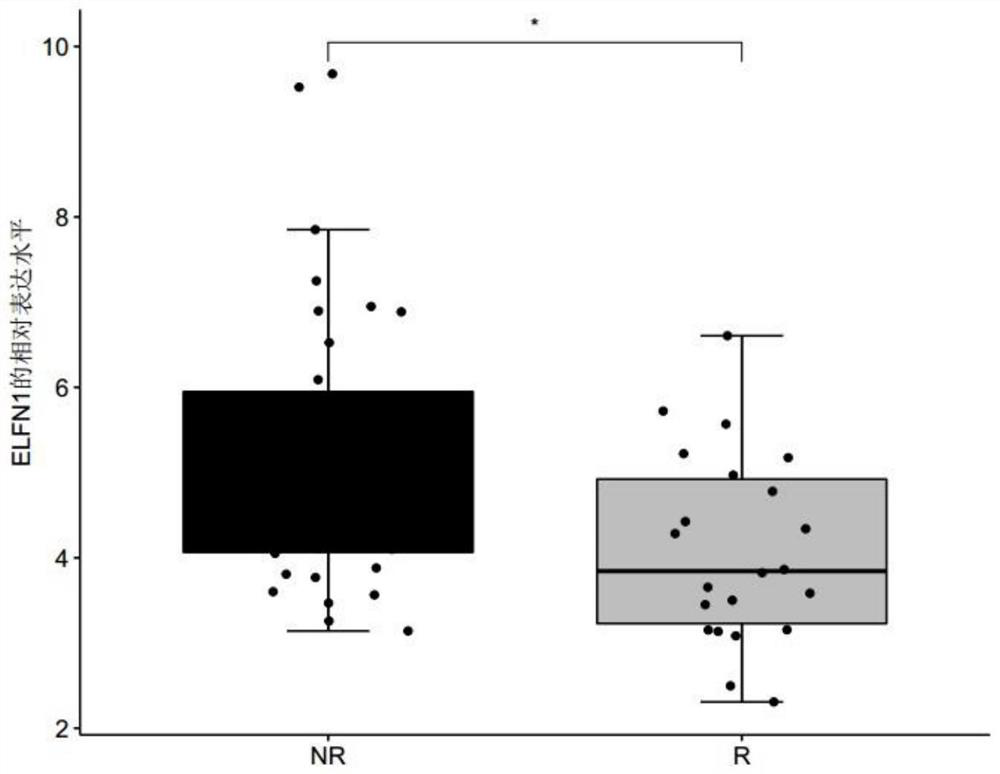
[0067] Example 1 Screening of genes related to the sensitivity of metastatic melanoma to immune drug MAGE-A3
[0068] 1. Data sources and screening methods
[0069] Patients with clinically diagnosed metastatic melanoma were collected, and none of these patients had received immunotherapy with MAGE-A3 before immunotherapy with MAGE-A3. Before the patients with metastatic melanoma collected above received the immune drug MAGE-A3 immunotherapy, the tumor biopsy tissues of the patients were collected, and the response group (R, responder) or non-response group ( Differentially expressed genes in tumor tissues of NR, non-responder) patients. The MAGE-A3 immunotherapy refers to administering the immune drug MAGE-A3 to patients with metastatic melanoma, and the immune drug MAGE-A3 refers to a substance obtained by dissolving recombinant MAGE-A3 protein in an immunostimulator (AS02B or AS15) . Among them, according to whether the metastatic melanoma is responsive / sensitive to the ...
Embodiment 2
[0073] Application of Differentially Expressed Genes Screened in Example 2 in Predicting Sensitivity of Melanoma to Immunotherapy Drug MAGE-A3
[0074] 1. Experimental method
[0075] For the genes differentially expressed between the responder group and the non-response group screened in Example 1, receiver operating characteristic (ROC) analysis was performed using the R package "pROC" (version 1.15.0), and the receiver operating characteristic (ROC) was calculated. The area under the characteristic curve (AUC) is used to evaluate the accuracy of a single differentially expressed gene, any combination of two differentially expressed genes, and the combination of three differentially expressed genes for predicting the sensitivity of melanoma to the immune drug MAGE-A3 treatment, wherein the The range of AUC value is 0-1;
[0076] Among them, when judging the diagnostic efficacy of a single differentially expressed gene for predicting the sensitivity of melanoma to the immuno...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com