New application of REGEND001 cell autotransfusion preparation
A cell and autologous technology, applied in the field of clinical medicine, can solve the problems of lack of alveolar structure, lack of conventional treatment methods, and failure to truly prevent fibrosis, etc., to achieve the effect of repairing lung damage and improving lung ventilation function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of autologous reinfusion preparation of REGEND001 cells
[0029] This embodiment provides a preparation process of clinical-grade autologous bronchial basal layer cells, which specifically includes the following steps:
[0030] The isolated active bronchi were collected for brush examination for later use. In this embodiment, the tissues located in two different parts of the bronchi were selected. The reason why two or more parts are selected is to ensure the success rate of separation.
[0031] Take the tissue digestion solution and stop solution for later use; 99v% of the tissue digestion solution is DMEM / F12, the rest is 1-20ng / mL DNase, 0.1-4mg / mL protease XIV and 10-200ng / mL trypsin; stop 90v% of the liquid is DMEM, and 10v% is FBS.
[0032] The above-mentioned brushed tissue is digested with the tissue digestion solution, and the digested tissue is terminated with the stop solution, and then the cells are collected. The cells after enzymat...
Embodiment 2
[0042] Example 2 Preparation of autologous reinfusion preparation of REGEND001 cells
[0043] This embodiment provides a preparation process of clinical-grade autologous bronchial basal layer cells, which specifically includes the following steps:
[0044] The isolated active bronchi were collected for brush examination for later use. In this embodiment, the tissues located in two different parts of the bronchi were selected. The reason why two or more parts are selected is to ensure the success rate of separation.
[0045] Take the tissue digestion solution and stop solution for later use; 99v% of the tissue digestion solution is DMEM / F12, the rest is 1-20ng / mL DNase, 0.1-4mg / mL protease XIV and 10-200ng / mL trypsin; stop 90v% of the liquid is DMEM, and 10v% is FBS.
[0046] The above-mentioned brushed tissue is digested with the tissue digestion solution, and the digested tissue is terminated with the stop solution, and then the cells are collected. The cells after enzymat...
Embodiment 3
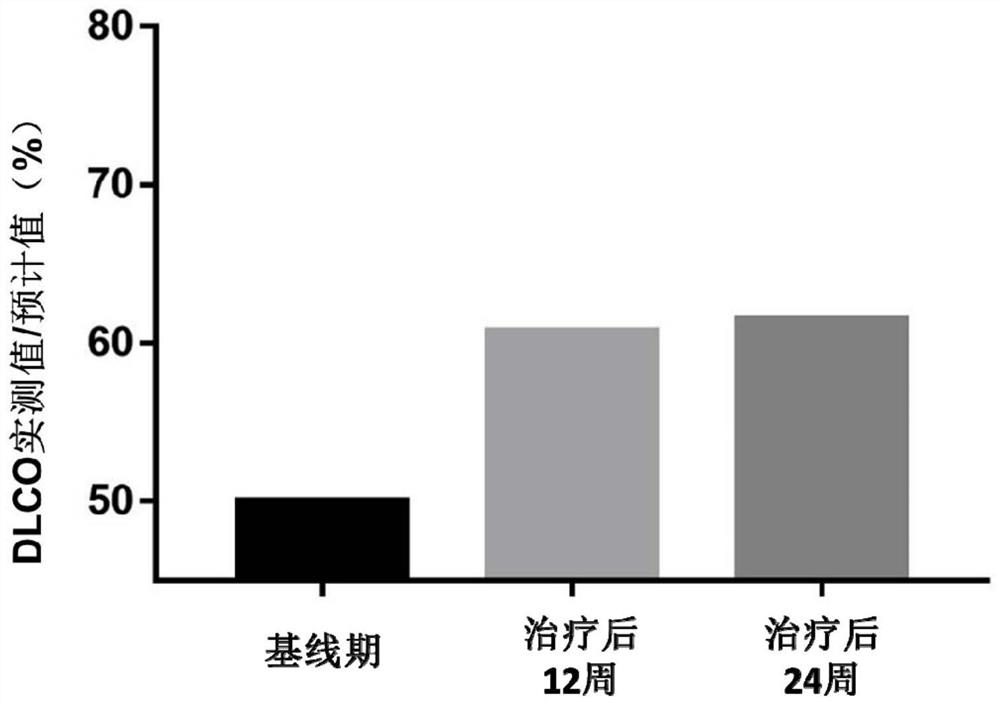
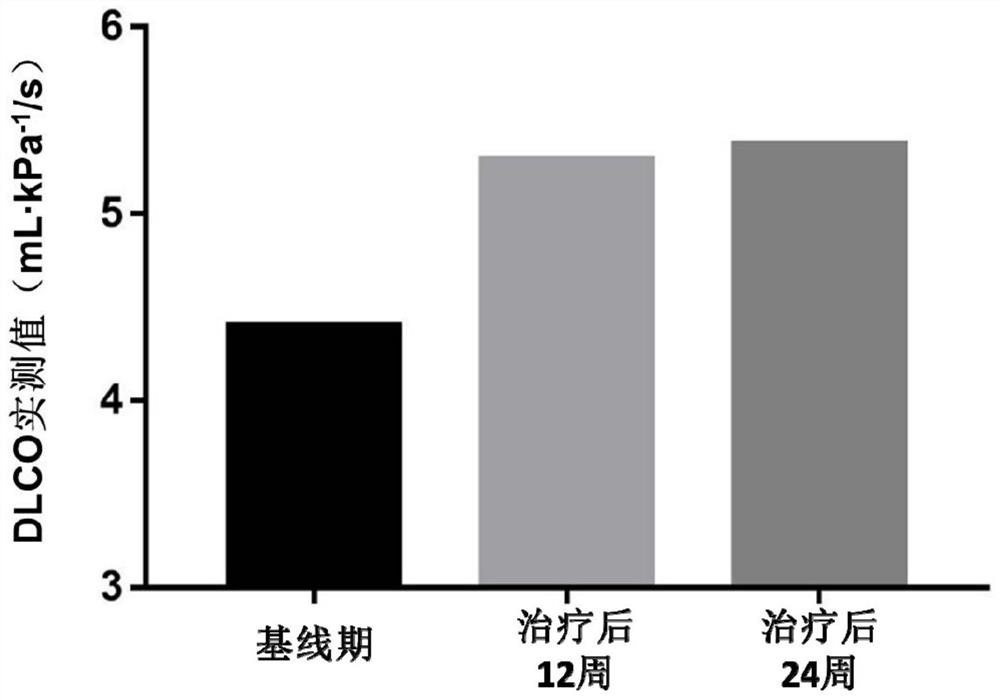
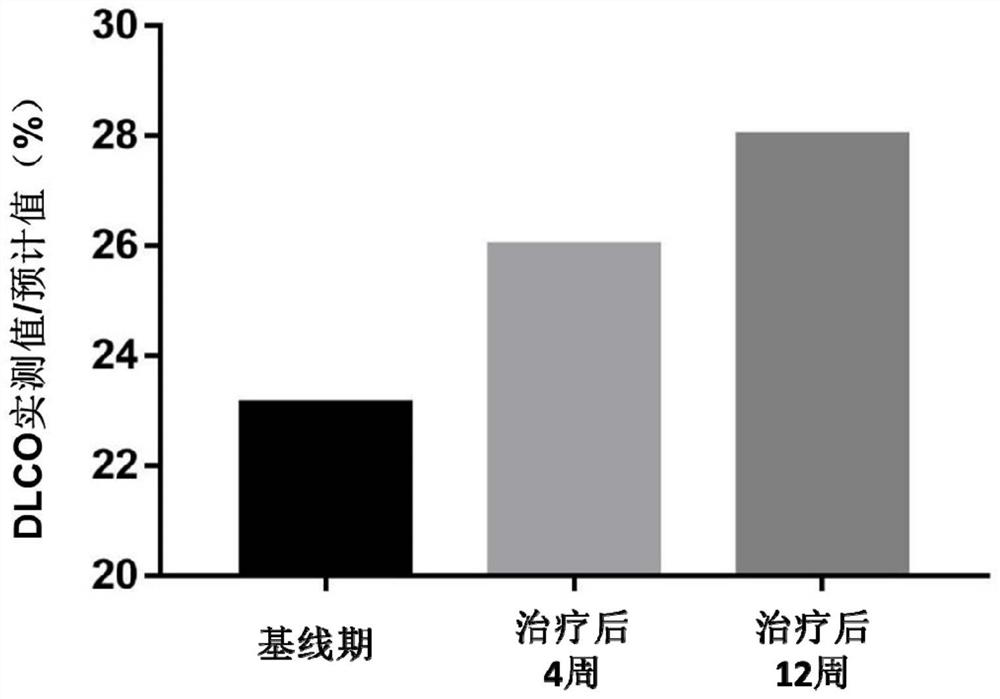
[0057]Implementation 3 REGEND001 cell autologous reinfusion preparation for the treatment of idiopathic pulmonary fibrosis
[0058] 1) Specific steps:
[0059] ①The reinfusion sites are the lingual lobe of the left lung, the lower lobe of the left lung and the middle lobe of the right lung, and the 14 lung subsegments (segmental bronchi) of the lower lobe of the right lung, which are respectively the lateral segmental bronchi of the right middle lobe, the medial segmental bronchi of the right middle lobe, Right lower lobe apical bronchi, right inner basal bronchi, right anterior basal bronchi, right outer basal bronchi, right posterior basal bronchi, left upper lingual bronchi, left lower lingual bronchi, Left lower lobe apical bronchi, left anterior basal bronchi, left inner basal bronchi, left outer basal bronchi, and left posterior basal bronchi. The bronchi of the upper lobe of the left lung and the upper lobe of the right lung are not reinfused;
[0060] ②Take 14mL of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com