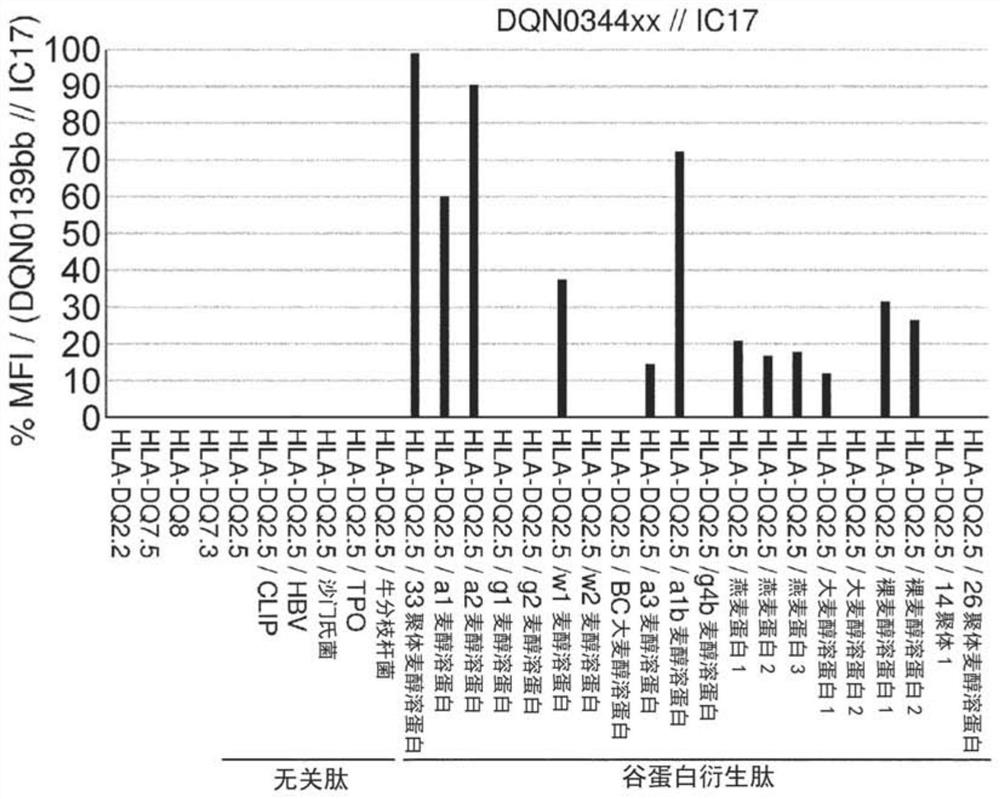
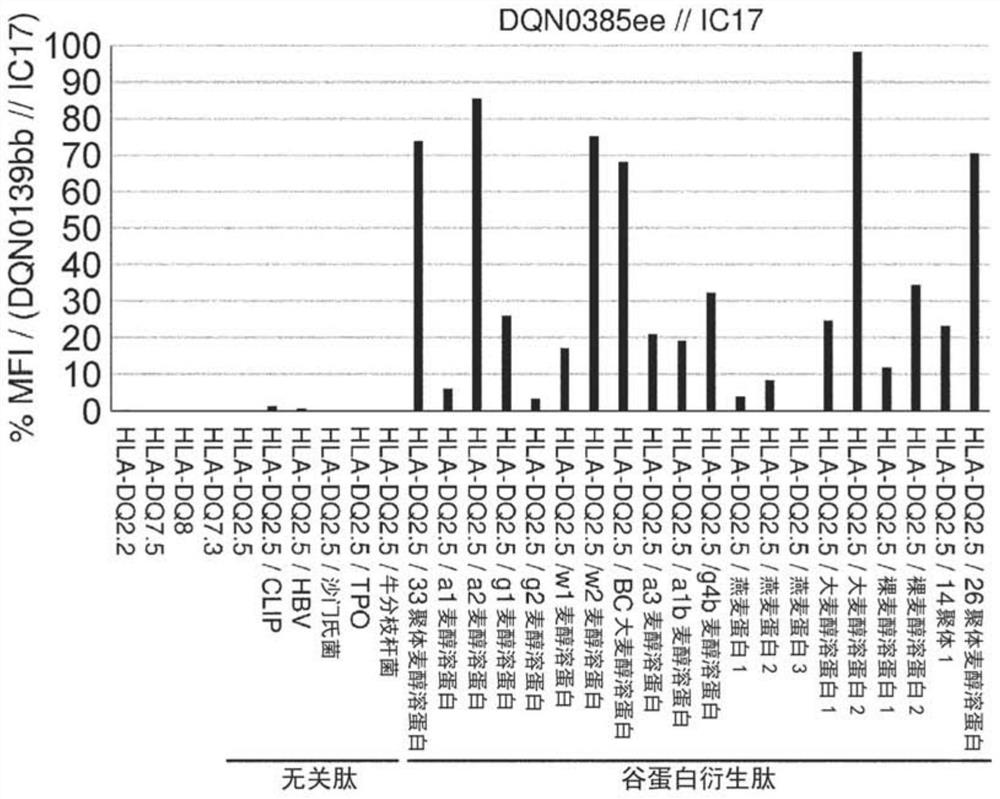
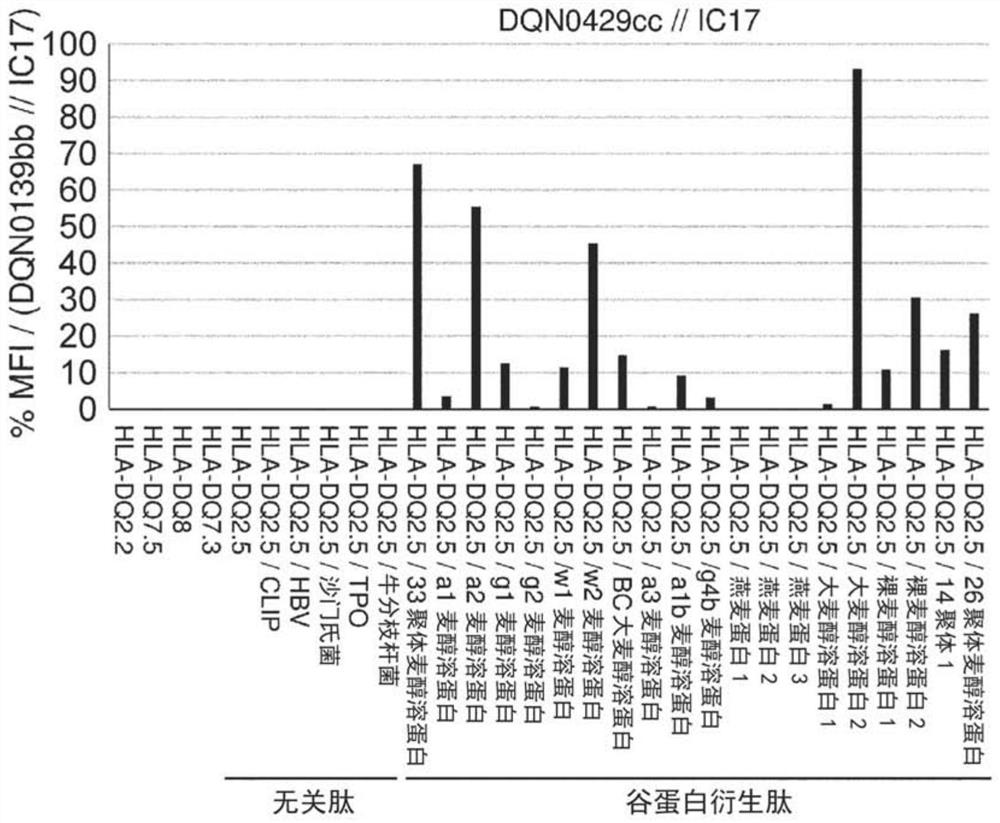
Anti-hla-dq2.5 antibody
A polymer and antigen-binding molecule technology, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, recombinant DNA technology, anti-plant immunoglobulin, etc., can solve the problem of completely eliminating gluten exposure, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0635] 1) Immunoconjugates
[0636] The invention also provides immunoconjugates comprising an anti-HLA-DQ2.5 antibody herein conjugated to one or more cytotoxic agents, such as chemotherapeutic agents or drugs, growth inhibitory agents, Toxins (eg protein toxins, enzymatically active toxins of bacterial, fungal, vegetable or animal origin, or fragments thereof), or radioactive isotopes.
[0637] In one embodiment, the immunoconjugate is an antibody-drug conjugate (ADC), wherein the antibody is conjugated to one or more drugs, including but not limited to maytansinoid (see U.S. Pat. Nos. 5,208,020, 5,416,064 and European Patent EP 0 425 235 B1); auristatins such as monomethyl auristatin drug moieties DE and DF (MMAE and MMAF) (see U.S. Patent Nos. 5,635,483 and 5,780,588 and 7,498,298); dorax Dolastatin; calicheamicin or derivatives thereof (see U.S. Pat. (1993); and Lode et al., Cancer Res. 58:2925-2928 (1998)); anthracyclines such as daunomycin or doxorubicin (see Kratz et...
Embodiment 1
[0670] Expression and purification of recombinant proteins
[0671] 1.1. Recombinant HLA-DQ2.5 / 33-mer gliadin peptide complex, HLA-DQ8 / gliadin peptide complex, HLA-DQ5.1 / DBY peptide complex, HLA-DQ2.2 / CLIP Expression and purification of peptide complexes, HLA-DQ7.5 / CLIP peptide complexes, HLA-DQ2.5 / γ2 gliadin peptide complexes, and HLA-DQ2.5 / BC hordein peptide complexes
[0672] Expression and purification of recombinant HLA-DQ2.5 / 33-mer gliadin peptide complex:
[0673] The sequences used for expression and purification are: HLA-DQA1*0501 (Protein Database Accession No. 4OZG) and HLA-DQB1*0201 (Protein Database Accession No. 4OZG), both of which have the CAMPATH-1H signal sequence: MGWSCIILFLVATATGVHS (SEQ ID NO :37). HLA-DQA1*0501 has a C47S mutation, a GGGG linker (SEQ ID NO: 38) and a c-fos leucine zipper sequence (PNAS, September 29, 1998; 95(20): 11828-33) and in HLA-DQA1 *Flag-Tag at the C-terminus of 0501. HLA-DQB1*0201 has a 33-mer gliadin peptide sequence: LQLQPF...
Embodiment 2
[0687] 2.1 Establishment of J.RT3-T3.5 cell line expressing D2 TCR
[0688] The D2 TCRα chain cDNA (SEQ ID NO: 97) was inserted into the expression vector pCXND3 (WO2008 / 156083). The D2 TCR β chain cDNA (SEQ ID NO: 49) was inserted into the expression vector pCXZD1 (US2009 / 0324589). Linearized D2 TCRα chain-pCXND3 and D2 TCRβ chain-pCXZD1 (1500 ng each) were simultaneously introduced into the J,RT3-T3,5 cell line by electroporation (LONZA, 4D-Nucleofector X). The transfected cells were then cultured in a medium containing geneticin and bleomycin (Zeocin), and then sorted using AriaIII (Becton Dickinson) to obtain a high-expressing cell population. Single cell cloning is then performed to obtain cells that highly express the desired D2 TCR molecule.
[0689] 2.2 Expression of HLA-DQ2.5, HLA-DQ2.2, HLA-DQ7.5, HLA-DQ8, HLA-DQ5.1, HLA-DQ6.3, HLA-DQ7.3, HLA-DR and HLA-DP Establishment of Ba / F3 cell line
[0690] HLA-DQA1*0501cDNA (IMGT / HLA accession number HLA00613), HLA-DQA1*0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com