Mucosal vaccine formulations
A technology of formulations, bioadhesives, applied in the field of formulations for mucosal application, capable of solving problems such as retention difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
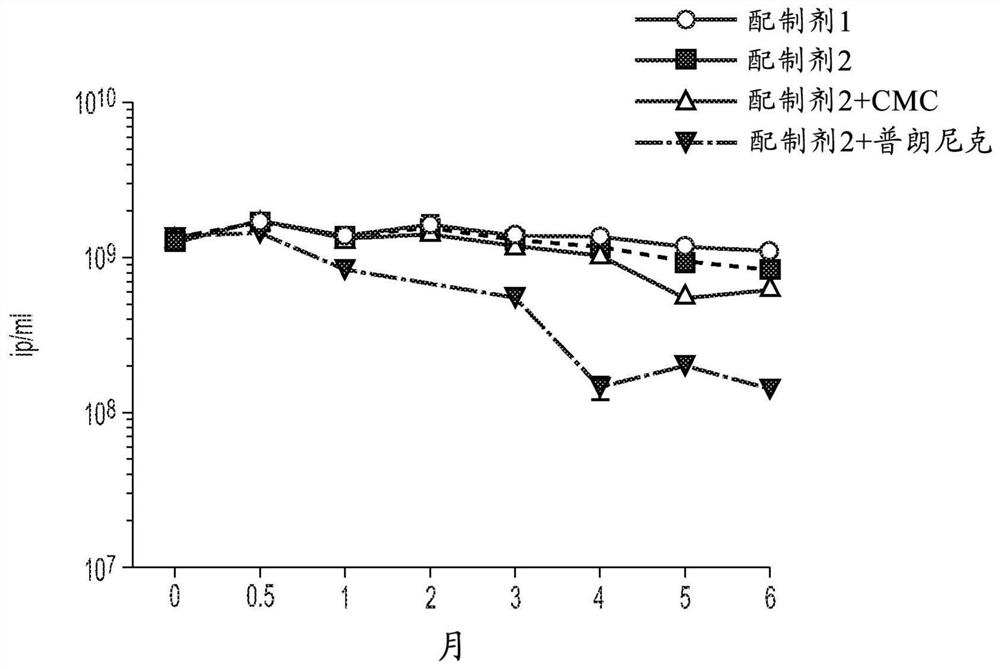
[0134] Example 1: In vitro stability of adenovirus in bioadhesive formulations
[0135] The stability of bioadhesive formulations of adenovirus at 4°C and 37°C and after freeze-thaw was tested in vitro. Stability was measured by qualitative PCR (qPCR) and with an infectivity assay detecting the adenovirus hexon protein in cultured cells. The effect of bioadhesive agents on adenovirus stability was evaluated using a genetically modified replication-deficient ChAd155 vector (ChAd155-RGco2) with deleted E1 / E4 gene regions and expressing codon-pair optimized rabies glycoprotein (G) (WO2018 / 104919).
[0136] Degradation of ChAd155 virions in various storage media was evaluated experimentally by measuring the infectivity of virus preparations over time at a controlled storage temperature of 4°C. Infectivity was determined in HEK293 cells using a hexon ELISA assay that measures the expression of viral hexon proteins following infection of cells. Stability is expressed as the ab...
Embodiment 2
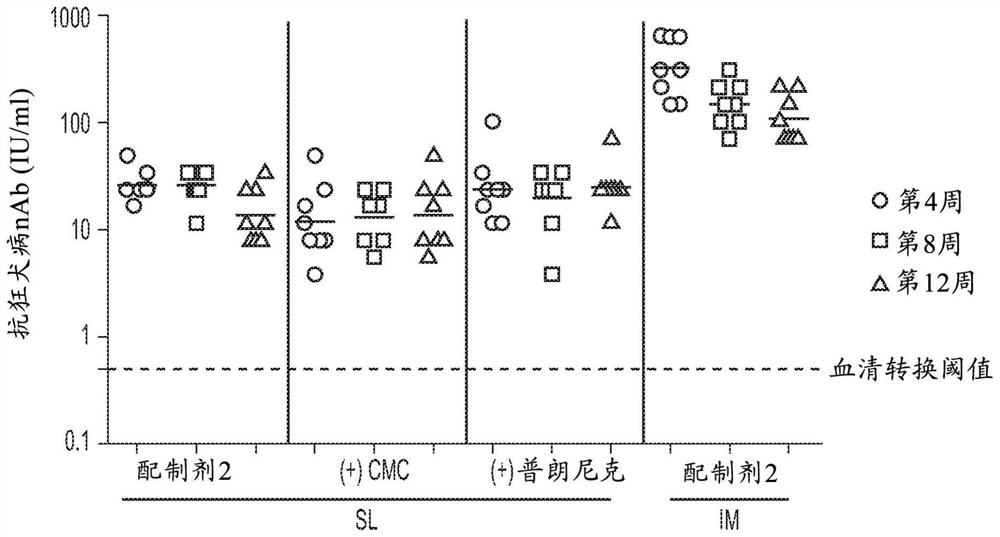
[0140] Example 2: In vivo immunogenicity of adenovirus in bioadhesive formulations
[0141] To determine the effect of bioadhesives on the immunogenicity of adenovirus, 1x10 9 vp ChAd155-RGco2 was formulated in Formulation 2, Formulation 2 with 1.5% CMC or Formulation 2 with 20% Poloxamer 407. 7ul was delivered sublingually to each of six Balb / c mice. As a control, with 1x10 in formulation 2 9 A group of mice were immunized intramuscularly with vp ChAd155-RGco2. Anti-rabies virus neutralizing antibody (VNA) titers in sera were determined by fluorescent antibody virus neutralization (FAVN) test at 4, 6 and 8 weeks after vaccination.
[0142] Such as figure 2 As shown in , anti-rabies VNA titers were comparable among the three sublingually immunized groups, indicating that the presence of CMC or Poloxamer 407 in the formulation had no negative effect on the immune potency of the rabies vaccine. All sublingually immunized mice had titers well above the seroconversion thre...
Embodiment 3
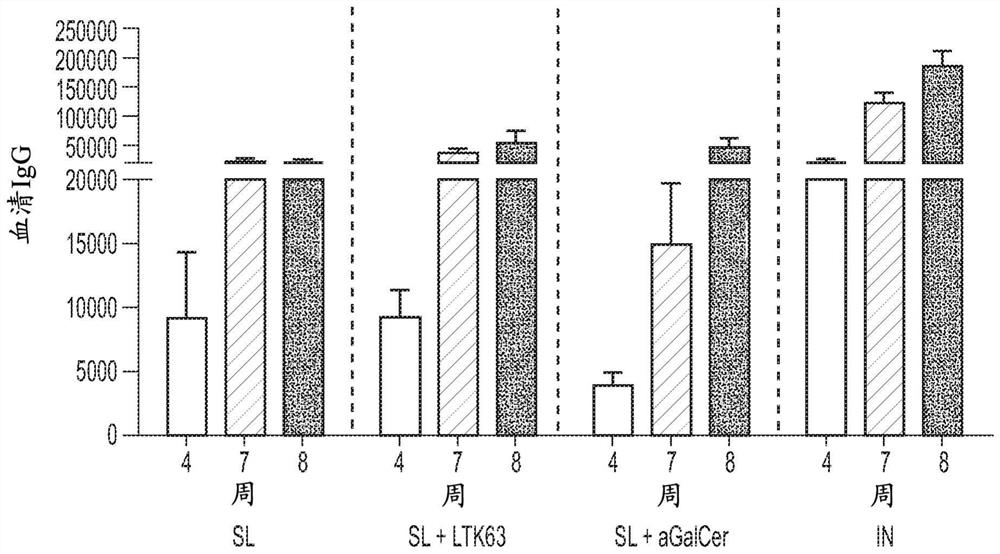
[0143] Example 3. Effect of known mucosal adjuvants on the immunogenicity of simian adenovirus
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| osmolarity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com