Administration of sting agonist and checkpoint inhibitors
A checkpoint and inhibitor technology, applied in the direction of anti-animal/human immunoglobulins, chemical instruments and methods, medical preparations containing active ingredients, etc., which can solve the problem of reducing the effect of radiotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0295] Example 1: In vivo tumor efficacy
[0296] General analysis method
[0297] Unless otherwise noted, obtained using Varian 300MHz 1 H NMR spectrum. HPLC was obtained on an Agilent 1100 Series and UPLC was obtained from Water Acuity Systems unless otherwise indicated.
[0298] Compound No. 14, as used in the following examples, can be synthesized according to the procedure described in Example 14 of PCT Publication No. WO 2018 / 100558.
[0299] General Experimental Conditions for Antitumor Efficacy in Mouse Tumor Models
[0300] Mouse Syngeneic Tumor Model
[0301] As specified below, the following homology models were utilized in each of Studies 1-5.
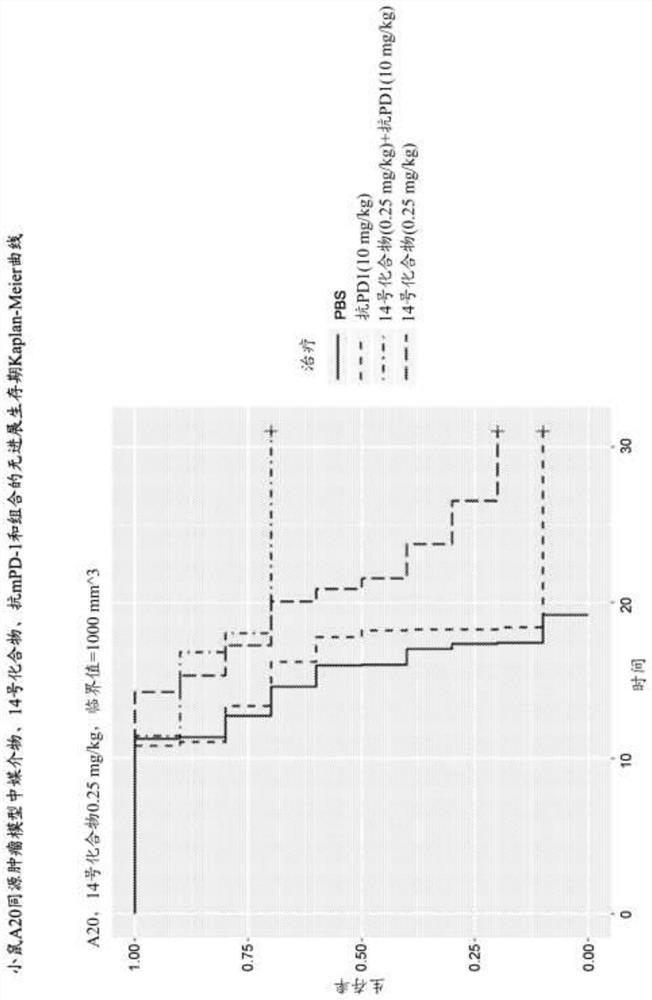
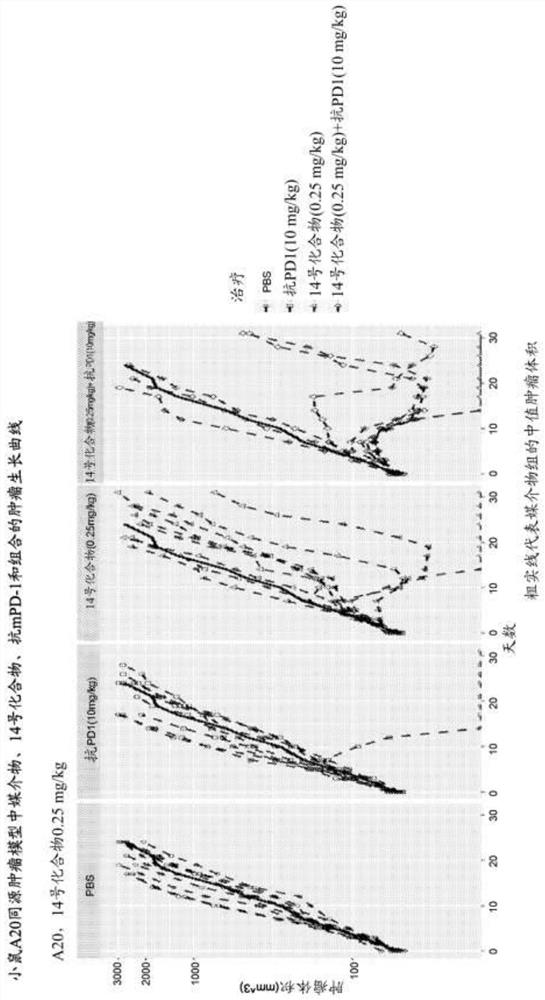
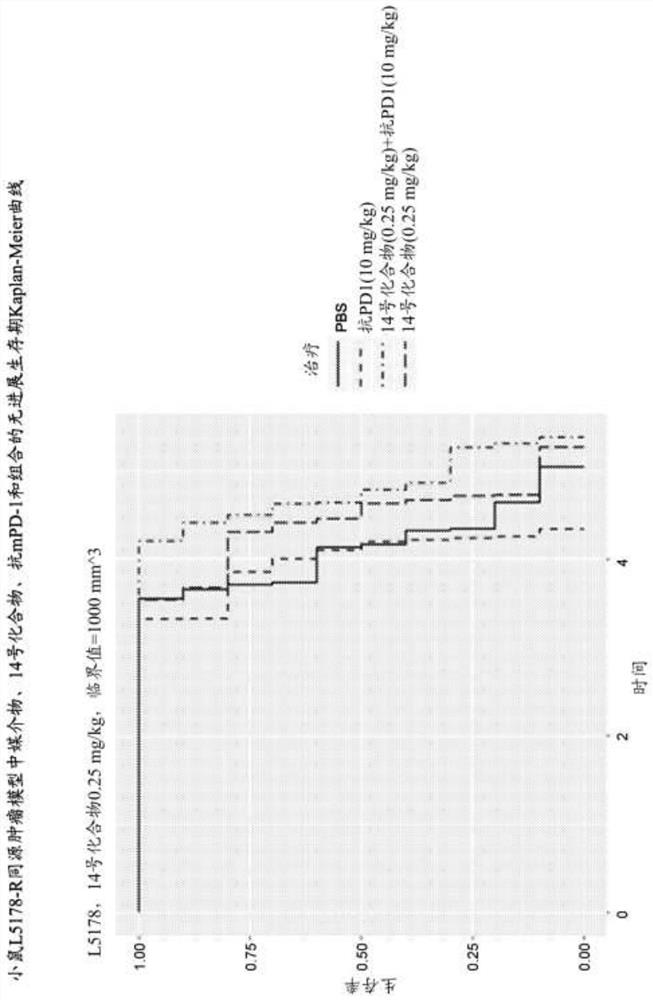
[0302] A20 Study 1: A20 is a mouse B-cell lymphoma cell line. By subcutaneously inoculating 0.4x10 6 A20 cells (cell suspension) were used to generate the A20 mouse syngeneic tumor model. When the average tumor volume reaches approximately 55 mm 3 , animals were randomly divided into a vehicle control group a...
Embodiment 2
[0337] Example 2: Evaluation of the clinical study of compound No. 14 combined with anti-PD-1 antibody in the treatment of patients with metastatic solid tumors
[0338] A Phase 1, open-label, parallel-assignment, dose-escalation study will be conducted to evaluate the safety, tolerability and tolerability of Compound 14 as a single agent (SA) and in combination with pembrolizumab in adult patients with metastatic solid tumors , pharmacokinetics (PK) and pharmacodynamics. This information will be used to independently assess the pharmacologically active dose (PAD) maximum tolerated dose (MTD) to determine the SA and the recommended second phase dose (RP2D) in combination with pembrolizumab. Based on discussion of the safety data by the investigator and the sponsor, dose escalation may be stopped after the determination of the PAD but before the determination of the MTD.
[0339] Approximately 100 patients will be enrolled in this study. Once enrolled, patients will be admini...
Embodiment 3
[0353] Example 3: Evaluation of the clinical study of compound No. 14 combined with anti-PD-1 antibody in the treatment of patients with metastatic solid tumors
[0354]A Phase 1, open-label, parallel-assignment, dose-escalation study will be conducted to evaluate the safety, tolerability and tolerability of Compound 14 as a single agent (SA) and in combination with pembrolizumab in adult patients with metastatic solid tumors , pharmacokinetics (PK) and pharmacodynamics. This information will be used to independently assess the pharmacologically active dose (PAD) maximum tolerated dose (MTD) to determine the SA and the recommended second phase dose (RP2D) in combination with pembrolizumab. Based on discussion of the safety data by the investigator and the sponsor, dose escalation may be stopped after the determination of the PAD but before the determination of the MTD.
[0355] Approximately 100 patients will be enrolled in this study. Once enrolled, patients will be adminis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com