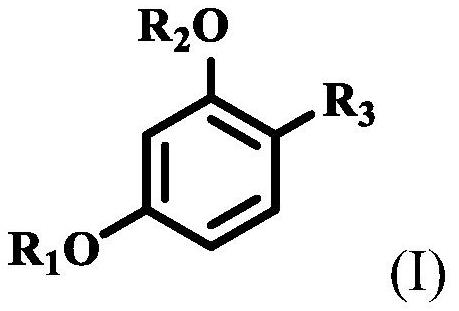
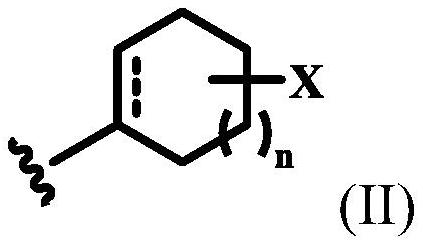
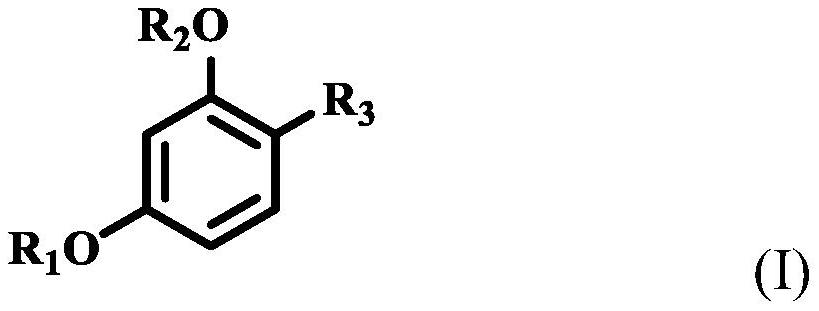
Stabilization of resorcinol compounds in cosmetic compositions
A technology of cosmetic composition and resorcinol, which is applied in the direction of cosmetics, cosmetic preparations, dressing preparations, etc., can solve the problems of instability and difficulty in preparation, and achieve the effect of improving storage/oxidation stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] Evaluation of signs of degradation and stabilization of 4-alkylresorcinols
[0162] color measurement
[0163] By dissolving 4-hexylresorcinol (200 mg) or 4-ethylresorcinol (200 mg) in deionized water: butanediol (4:1; 20 ml) to produce a clear, colorless homogeneous solution, to prepare samples for color evaluation. A portion (5 ml) of each solution was added to 20 ml scintillation vials containing nicotinamide (B3) (50 mg), AceMet (acetylmethionine) (50 mg) or B3 (50 mg) + AceMet (50 mg), and Monitor at room temperature (-20-22°C). After 2 weeks, the color of each solution was assessed visually and L*a*b* measurements were determined experimentally using a Labscan XE instrument (Hunter Associates Labs Inc., Reston, VA) and processed with Universal software (version 4.10). The L* parameter measures darkness and lightness and ranges from black (L*=0) to white (L*=100). The a* parameter measures color content and intensity from green (a*0). The b* parameter also...
Embodiment 2
[0174] Example 2. ER and AceMet
[0175] Topical cosmetic compositions within the scope of the present invention are prepared.
[0176] The base formulations shown in the table below were prepared by heating the Phase A ingredients to 70 to 85°C with agitation. Heat Phase B ingredients to 70 to 85°C in a separate vessel with stirring. Then, add Phase A to Phase B while maintaining both phases at 70 to 85°C. The mixture was stirred at 70 to 85°C for at least 15 minutes, then cooled. Add phase C ingredients at 50°C, followed by phase D ingredients at 40°C.
[0177] Table 2. Base formulations
[0178]
[0179]
Embodiment 3
[0181] Additional cosmetic compositions falling within the scope of the present invention were prepared as shown in the table below.
[0182] table 3
[0183]
[0184]
[0185] The composition was prepared as follows:
[0186] 1. Heat Phase A to 80°C while mixing.
[0187] 2. Heat Phase B to 75°C in a separate container while mixing.
[0188] 3. Add B to A and mix for 15 minutes with heat maintained at 70-80°C; then turn off heat and continue mixing for another 15 minutes.
[0189] 4. Add Phase C and mix for 10 minutes at 50°C.
[0190] 5. Add Phase D and mix for 10 minutes at 40°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com