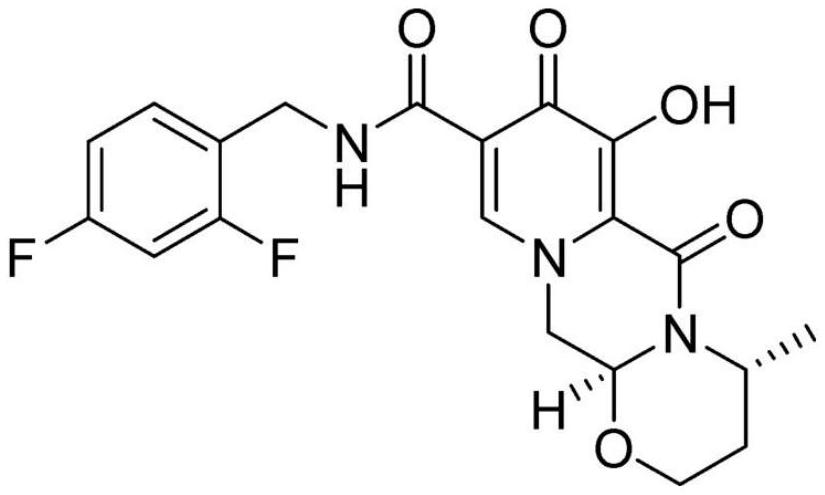
Preparation method of dolutegravir
A technology of dolutegravir and concentration, which is applied in the field of preparation of dolutegravir, can solve the problems of affecting bioavailability and poor solubility, and achieve the effects of excellent ultrafine effect, lower temperature, and excellent dispersion and stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) In the reaction kettle, add the solvent DMF, dissolve the original powder of dolutegravir in DMF, and prepare the DMF solution of dolutegravir, wherein the concentration of dolutegravir is 30mg / ml.
[0028] (2) In another reaction kettle, prepare an aqueous solution of polyvinyl alcohol PVA1788 with a concentration of 1%.
[0029] (3) Turn on the strong stirring of the polyvinyl alcohol aqueous solution, control the temperature at 0°C, and quickly pump the DMF solution of dolutegravir into the liquid level of the polyvinyl alcohol aqueous solution through the high-pressure delivery pump, and the flow rate at the outlet pipe greater than 3 m / s. Continue vigorous stirring for 60 min. Obtain dolutegravir suspension. Wherein the volume ratio of polyvinyl alcohol aqueous solution to the DMF solution of dolutegravir is 5:1.
[0030] (4) The suspension is subjected to solid-liquid separation, and the dolutegravir powder can be obtained after drying. The obtained dolute...
Embodiment 2
[0032] (1) In the reaction kettle, add the solvent DMF, dissolve the original powder of dolutegravir in DMF, and prepare the DMF solution of dolutegravir, wherein the concentration of dolutegravir is 70mg / ml.
[0033] (2) In another reaction kettle, prepare an aqueous solution of polyvinyl alcohol PVA2488 with a concentration of 3%.
[0034] (3) Turn on the strong stirring of the polyvinyl alcohol aqueous solution, control the temperature at 20°C, and quickly pump the DMF solution of dolutegravir into the liquid level of the polyvinyl alcohol aqueous solution through the high-pressure delivery pump, and the flow rate at the outlet pipe greater than 3 m / s. Continue vigorous stirring for 30 min. Obtain dolutegravir suspension. Wherein the volume ratio of polyvinyl alcohol aqueous solution to the DMF solution of dolutegravir is 50:1.
[0035] (4) The suspension is subjected to solid-liquid separation, and the dolutegravir powder can be obtained after drying. The obtained dolu...
Embodiment 3
[0037] (1) In the reaction kettle, add the solvent DMF, dissolve the original powder of dolutegravir in DMF, and prepare the DMF solution of dolutegravir, wherein the concentration of dolutegravir is 50mg / ml.
[0038] (2) In another reaction kettle, prepare an aqueous solution of polyvinyl alcohol PVA1788 with a concentration of 2%.
[0039] (3) Turn on the strong stirring of the polyvinyl alcohol aqueous solution, control the temperature at 10°C, and quickly pump the DMF solution of dolutegravir into the liquid level of the polyvinyl alcohol aqueous solution through the high-pressure delivery pump, and the flow rate at the outlet pipe greater than 3 m / s. Continue vigorous stirring for 45 min. Obtain dolutegravir suspension. Wherein the volume ratio of polyvinyl alcohol aqueous solution to the DMF solution of dolutegravir is 25:1.
[0040] (4) The suspension is subjected to solid-liquid separation, and the dolutegravir powder can be obtained after drying. The obtained dolu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com