Anthryl molecular junction with photoelectric detection function and preparation method and application thereof
An anthracene-based molecular junction and anthracene-based molecule technology, which is applied in the field of molecular electronics, can solve problems such as less molecular photodetectors, and achieve the effects of short preparation time, stable growth of self-assembled molecular films, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
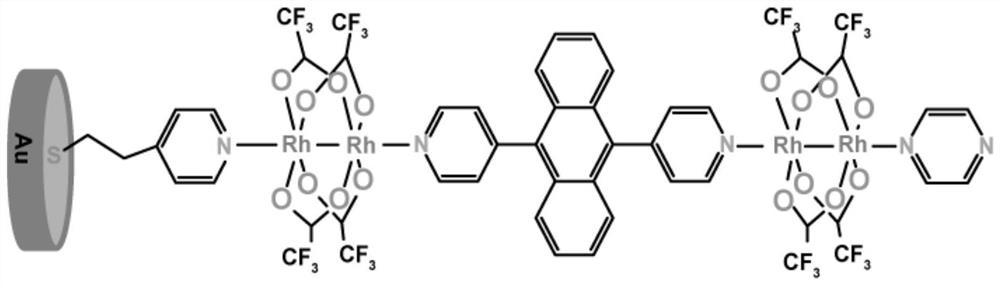
[0031] Embodiment 1: The anthracene-based molecular junction (ABP-SM) with photodetection function of this embodiment
[0032] in [Rh 2 (O 2 CCF 3 ) 4 ] is a Lewis acid, 9,10-bis(4-pyridyl)anthracene is a photosensitive molecule, and pyrazine is a pyridyl bidentate organic ligand.
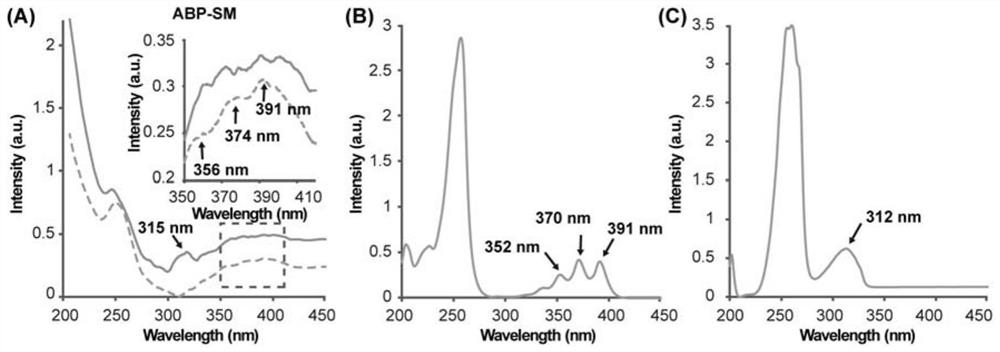
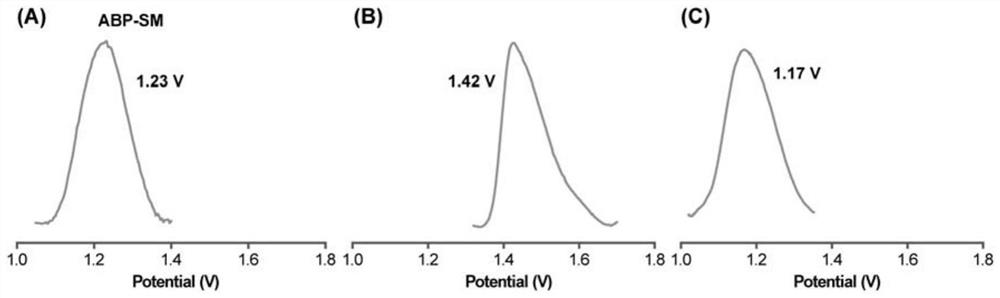
[0033] The steps are as follows: at room temperature, place the gold substrate electrode in 0.1 mmol / L 4-mercaptopyridine ethanol solution and soak for 1 hour to prepare 4-mercaptopyridine SAMs. SAMs were rinsed with ethanol and washed with N 2 After purging and drying; it was transferred to 0.2 mmol / L rhodium trifluoroacetate dimer [Rh 2 (O 2 CCF 3 ) 4 ] ethanol solution, and soak for 1 hour at low temperature (2 (O 2 CCF 3 ) 4 ] soak in ethanol solution for 5 min, soak in pyrazine at room temperature for 5 min, rinse with ethanol after each soak and wash with N 2 After drying, the anthracene molecular junction (ABP-SM) can be obtained, and its structure is as follows figure 1 shown. ...
Embodiment 2
[0037] Example 2: Self-assembly of molecular junctions (ABP-SM) at different molar concentration ratios
[0038] The anthracene molecular junction (ABP-SM) with photodetection function in this embodiment. in [Rh 2 (O 2 CCF 3 ) 4 ] is a Lewis acid, 9,10-bis(4-pyridyl)anthracene is a photosensitive molecule, and pyrazine is a pyridyl bidentate organic ligand. Proceed as follows:
[0039] At room temperature, the gold substrate electrode was placed in 0.2mmol / L 4-mercaptopyridine ethanol solution and soaked for 1 hour to prepare 4-mercaptopyridine SAMs. SAMs were rinsed with ethanol and washed with N 2 After purging and drying; it was transferred to 0.6 mmol / L rhodium trifluoroacetate dimer [Rh 2 (O 2 CCF 3 ) 4 ] ethanol solution, and soak for 1 hour at low temperature (2 (O 2 CCF 3 ) 4 ] soak in ethanol solution for 5 min, soak in pyrazine at room temperature for 5 min, rinse with ethanol after each soak and wash with N 2 After drying, the anthracene molecular knot...
Embodiment 3
[0040] Example 3: Self-assembly of molecular junctions (ABP-SM) at different molar concentration ratios
[0041] The anthracene molecular junction (ABP-SM) with photodetection function in this embodiment. in [Rh 2 (O 2 CCF 3 ) 4 ] is a Lewis acid, 9,10-bis(4-pyridyl)anthracene is a photosensitive molecule, and pyrazine is a pyridyl bidentate organic ligand. Proceed as follows:
[0042] At room temperature, the gold substrate electrode was placed in 0.2mmol / L 4-mercaptopyridine ethanol solution and soaked for 1 hour to prepare 4-mercaptopyridine SAMs. SAMs were rinsed with ethanol and washed with N 2 After purging and drying; it was transferred to 1 mmol / L rhodium trifluoroacetate dimer [Rh 2 (O 2 CCF 3 ) 4 ] ethanol solution, and soak for 1 hour at low temperature (2 (O 2 CCF 3 ) 4 ] soak in ethanol solution for 5 min, soak in pyrazine at room temperature for 5 min, rinse with ethanol after each soak and wash with N 2 After drying, the anthracene molecular knot (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com