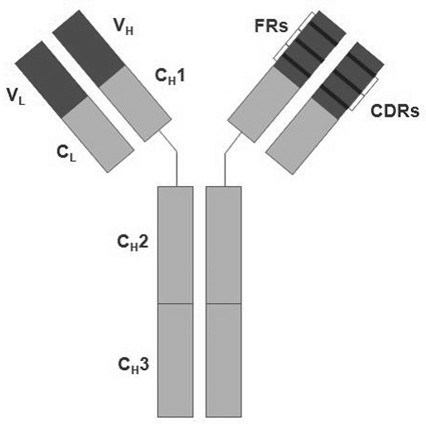
Anti-prostatic acid phosphatase antibody and use thereof
A technology of acid phosphatase and prostate, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0190] The preparation method of the nucleic acid is a conventional preparation method in the field, preferably, comprising the following steps: obtaining a nucleic acid molecule encoding the above-mentioned protein by gene cloning technology, or obtaining a nucleic acid molecule encoding the above-mentioned protein by artificial full sequence synthesis .
[0191] Those skilled in the art know that substitutions, deletions, alterations, insertions or additions can be appropriately introduced into the base sequence encoding the amino acid sequence of the above-mentioned proteins to provide a homolog of a polynucleotide. Homologs of the polynucleotides of the present invention can be prepared by substituting, deleting or adding one or more bases of the gene encoding the protein sequence within the range that maintains the activity of the antibody.
[0192] carrier
[0193] The present invention also provides a recombinant expression vector comprising the nucleic acid.
[0194]...
Embodiment 1
[0252] Example 1: Preparation and screening of anti-PAP antibody monoclonal cell lines
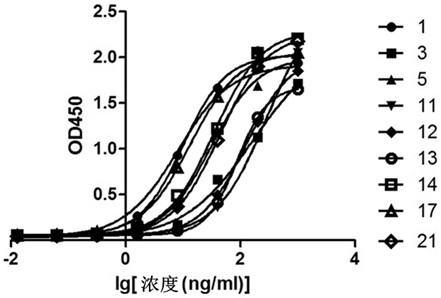
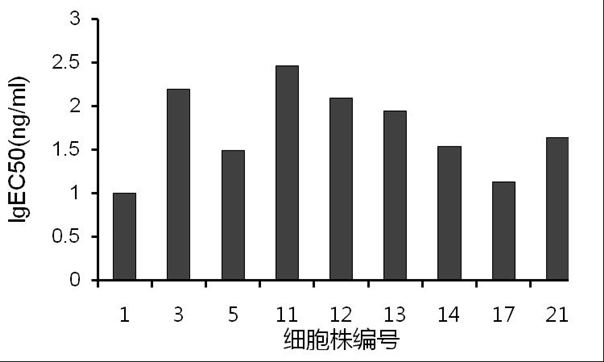
[0253] BalB / c mice were immunized multiple times with PAP-GM-CSF protein (refer to the patent application of the inventor for the preparation method: preparation method of prostatic acid phosphatase / granular-macrophage colony-stimulating factor, patent application number: 201810073775.X) , spleen cells of immunized mice and SP2 / 0 myeloma cells were fused to prepare hybridoma cells. By ELISA and monoclonalization, 9 monoclonal cell lines were finally screened, and their ELISA binding curves and ED50 were determined. value.
[0254] (1) Materials and instruments
[0255] 1. Mice: BalB / c
[0256] 2. Myeloma cells: SP2 / 0 (ATCC)
[0257] 3. Immunogen: PAP-GM-CSF protein (Shanghai Huidun Biotechnology Co., Ltd., 1mg / ml)
[0258] 4. Cell growth medium: 1640+10%FBS(Gibco)+OPI Media Supplement(Sigma)
[0259] 5. Detection antibody: M-HRP-anti-mouse IgG
[0260] 6. Cell fusion instrument: BTX ...
Embodiment 2
[0276] Example 2: Preparation of the PAP antibody of the present invention
[0277] experimental method:
[0278] The cell line with high expression of anti-PAP antibody obtained in Example 1 was then recovered, cultured and transferred to finally obtain a culture supernatant with high expression of anti-PAP antibody.
[0279] Cell culture supernatants were collected by centrifugation, pH adjusted with buffer A (20 mM PBS, pH 7.0), and loaded onto an affinity chromatography column that had been equilibrated with buffer A. After loading, use buffer A to wash the column to the A280 value and conductance back to the baseline, use 100% buffer B (20mM citric acid, pH 2.7) to elute in one step, monitor the eluent A280 value, and collect the elution fractions. The product was initially purified for anti-PAP-GM-CSF antibody.
[0280] The pH of the preliminary purified product was adjusted to 5.0-6.0 with buffer C (10 mM citric acid, pH 5.5) and loaded onto an equilibrated cation exc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com