T cell receptors and methods of use thereof
A technology of cell receptors and nucleic acid molecules, applied in animal cells, vertebrate cells, genetically modified cells, etc., can solve problems that hinder the comprehensive analysis of anti-tumor T cell specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0235] Example 1 - Method
[0236] cell
[0237] Peripheral mononuclear cells were obtained via density gradient centrifugation (Ficoll-Paque PLUS, GE Healthcare Life Sciences, Marlborough, MA). The K562 cell line is an erythroleukemic cell line with defective HLA class I / II expression. A K562-based artificial APC (aAPC) expressing individual HLA class I genes individually as a single HLA allele in combination with CD80 and CD83 has been previously reported (Butler et al., PloS One 7, e30229 (2012). Jurkat 76 The cell line is a T-cell leukemia cell line lacking expression of endogenous TCR, CD4 and CD8. Jurkat 76 / CD4 cells were produced by retroviral transduction of human CD4 gene. HEK293T cells and melanoma cell lines were supplemented with 10 %FBS and 50 μg / ml gentamicin (Thermo Fisher Scientific, Waltham, MA) in DMEM. K562 and Jurkat 76 cell lines were cultured in RPMI 1640 supplemented with 10% FBS and 50 μg / ml gentamicin .
[0238] peptide
[0239] MAGE-A2 108-127 S...
Embodiment 2
[0259] Example 2 - MAGE-A2 108-127 Characterization of TCR
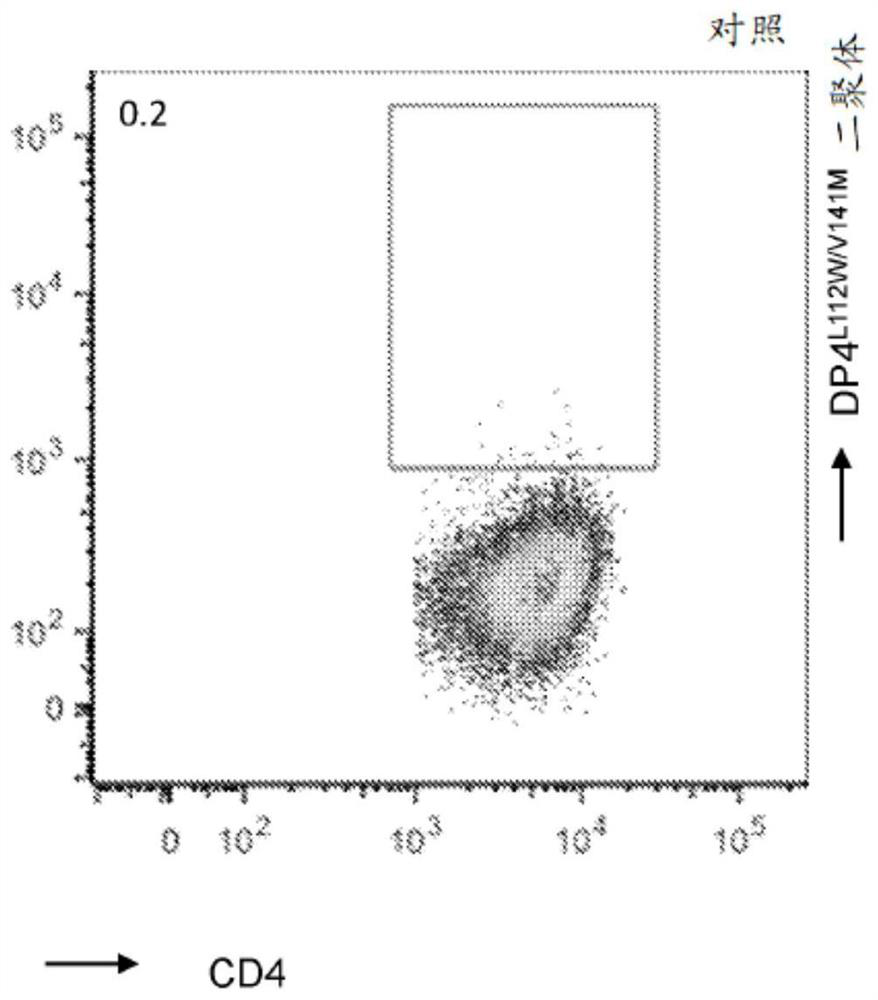
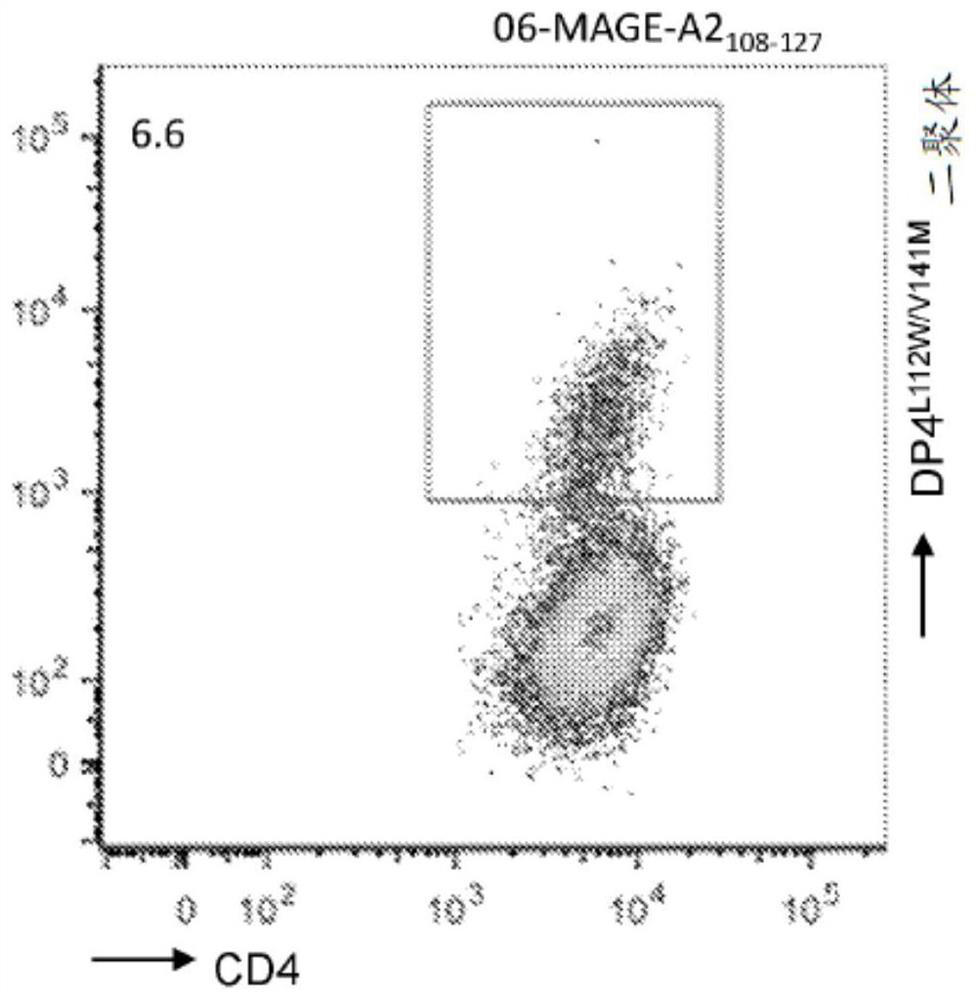
[0260] from six DP4 + Primary CD4 isolated from a patient with melanoma + T cells were stimulated only once with DP4-aAPC pulsed with peptide fragments (108-127) of MAGE-A2 and cognate DP4 L112W / V141M Dimer staining. To avoid potential in vitro priming, weak stimulation conditions were used. Discovery of MAGE-A2 by dimer staining 108-127 Immunogenic (data not shown).
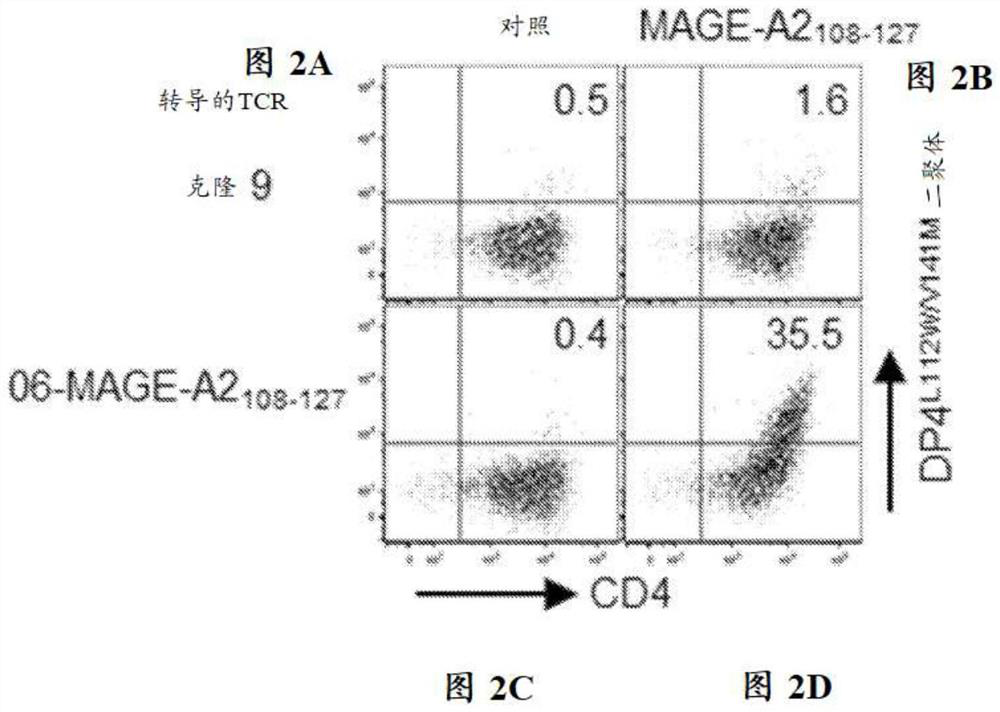
[0261] In order to verify the dimer staining results, MAGE-A2 was cloned from dimer-positive T cells 108-127 Specific DP4-restricted TCR gene ( Figures 1A-1B and Table 6). when human CD4 + During clonotypic recombination in TCR-deficient T cells, MAGE-A2 108-127 TCR is cognate DP4 L112W / V141M Dimers were successfully stained ( Figures 2A-2D ), and function in a DP4-restricted and antigen-specific manner ( image 3 ).
[0262] TCR 03-MAGE-A2 108-127 Ability to recognize homologous peptides endogenously processed and presented by DP4 ( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com