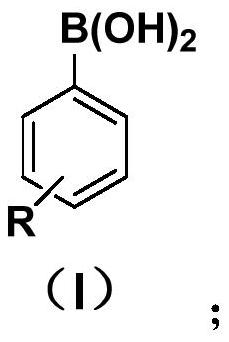
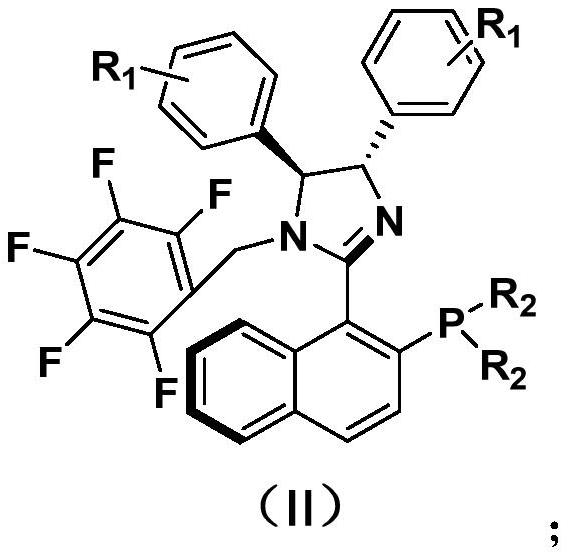
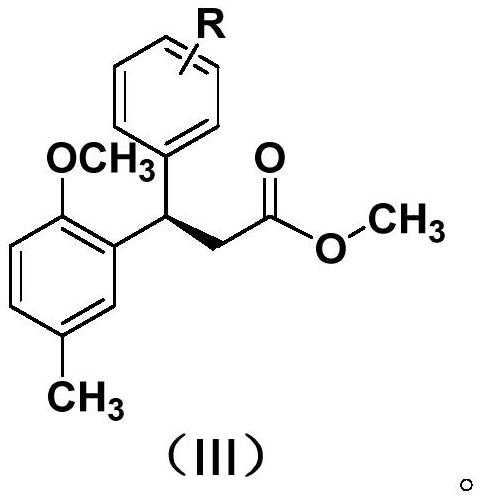
Method for preparing (R)-2, 3-diaryl substituted methyl propionate compound
A technology for methyl propionate and compounds, which is applied in the field of preparation of -2,3-diaryl substituted methyl propionate compounds, can solve problems such as difficulties, expensive catalysts and chiral resolution, and achieve low catalyst consumption , good yield and enantioselectivity, and the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation method of (R)-2,3-diaryl substituted methyl propionate compound, specifically comprises the following steps:
[0028] In a dry 15mL pressure-resistant reaction tube, add 103mg of 2-methoxy-4-methyl-cinnamic acid methyl ester (CAS: 86761-35-5), 78mg of phenylboronic acid, 2.0mg of cuprous chloride, 7.8mg P, N-type chiral ligand, 138mg potassium carbonate and 2.5ml tetrahydrofuran. The reaction was stirred at 50°C for 2 hours under nitrogen. After the reaction was completed, it was cooled to room temperature and directly passed through a silica gel column (volume ratio of petroleum ether to ethyl acetate was 15:1) to obtain 108 mg of product with a yield of 76% and ee of 89%. The reaction process is shown in the following formula:
[0029]
[0030] Carry out nuclear magnetic resonance and mass spectrometry to the product prepared in this embodiment:
[0031] 1 H NMR (400MHz, CDCl 3 ): δ=7.40(t, J=7.6Hz, 2H), 7.29(d, J=7.6Hz, 2H), 7.27(d, J=7.6Hz, 1H...
Embodiment 2
[0035] The preparation method of (R)-2,3-diaryl substituted methyl propionate compound, specifically comprises the following steps:
[0036] In a dry 15mL pressure-resistant reaction tube, add 103mg of 2-methoxy-4-methyl-cinnamic acid methyl ester (CAS: 86761-35-5), 97mg of phenylboronic acid, and 2.0mg of cuprous chloride, 7.8mg P, N-type chiral ligand, 138mg potassium carbonate and 2.5ml tetrahydrofuran. The reaction was stirred at 50°C for 2 hours under nitrogen. After the reaction was completed, it was cooled to room temperature, and directly passed through a silica gel column (the volume ratio of petroleum ether to ethyl acetate was 15:1), and 114 mg of the product was obtained, with a yield of 73%, and ee of 91%. The reaction process is shown in the following formula:
[0037]
[0038] Carry out nuclear magnetic resonance and mass spectrometry to the product prepared in this embodiment:
[0039] 1 H NMR (400MHz, CDCl 3 ): δ=7.38(t, J=7.6Hz, 2H), 7.31(d, J=7.6Hz, 2...
Embodiment 3
[0043] The preparation method of (R)-2,3-diaryl substituted methyl propionate compound, specifically comprises the following steps:
[0044] In a dry 15mL pressure-resistant reaction tube, add 103mg of 2-methoxy-4-methyl-cinnamic acid methyl ester (CAS: 86761-35-5), 93mg of 4-fluorophenylboronic acid, 2.0mg of chloride Cuprous, 7.8mg P, N-type chiral ligand, 138mg potassium carbonate and 2.5ml tetrahydrofuran. The reaction was stirred at 50°C for 2 hours under nitrogen. After the reaction was completed, it was cooled to room temperature, and directly passed through a silica gel column (the volume ratio of petroleum ether to ethyl acetate was 15:1), and 106 mg of product was obtained, with a yield of 70%, and ee of 89%. The reaction process is shown in the following formula:
[0045]
[0046] Carry out nuclear magnetic resonance and mass spectrometry analysis to the product prepared in this embodiment:
[0047] 1 H NMR (400MHz, CDCl 3): δ=7.43(t, J=7.6Hz, 2H), 7.37(d, J=...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap