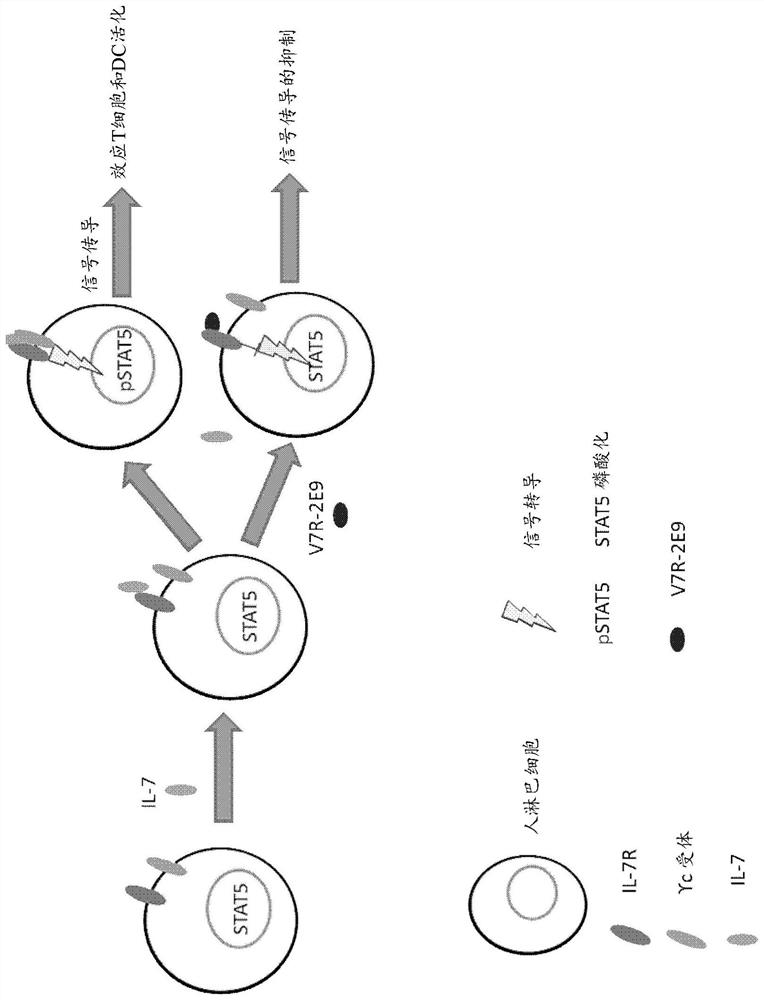
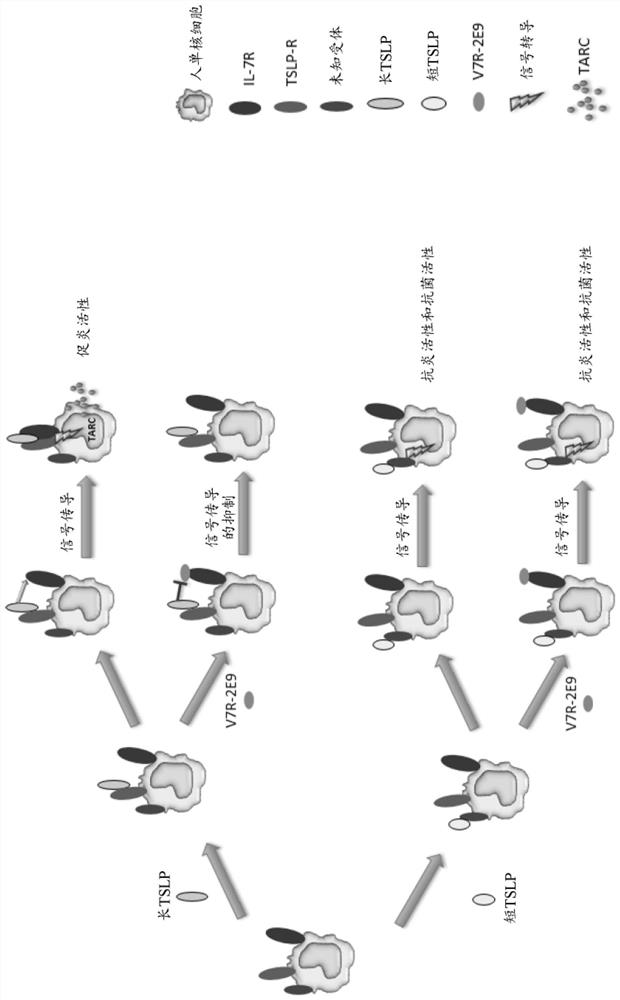
Polypeptides
A sequence and identity technology, applied in the field of peptides, can solve the problems of undetectability, failure, instability, etc., and achieve the effect of inhibiting the inflammatory process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
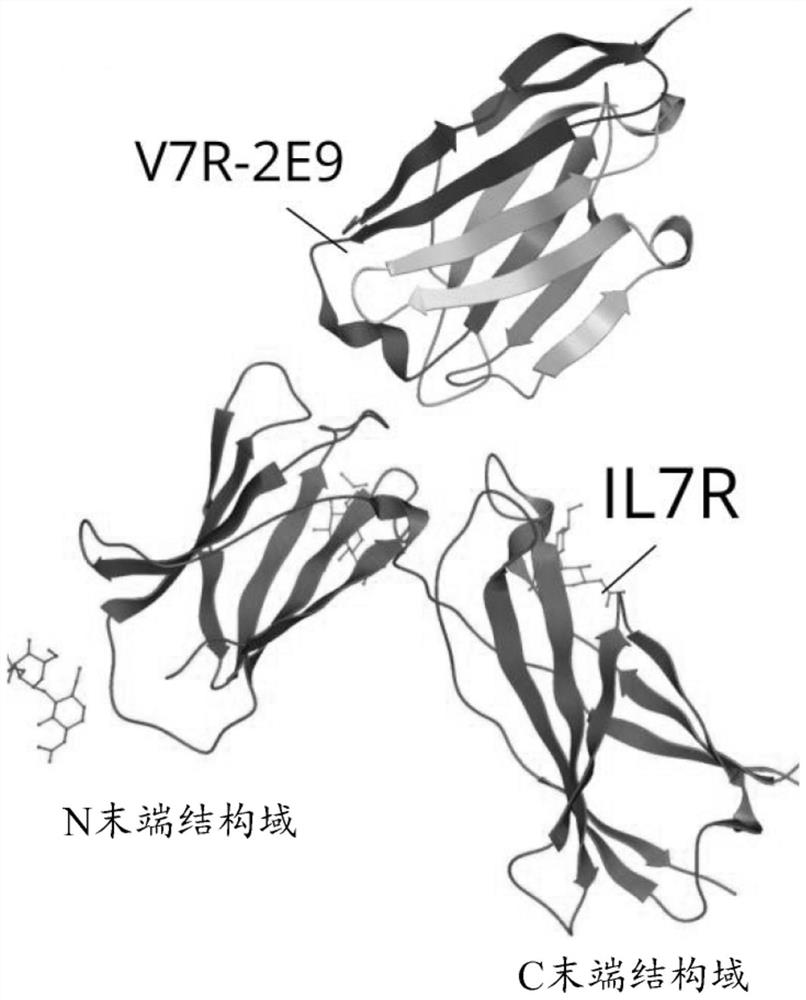
[0263] Example 5 describes in detail the epitope modeling work performed on the polypeptide V7R-2E9 of the present invention. This work indicates that V7R-2E9 binds to the following residues of IL-7Rα. Residues covering particularly significant regions at the interface are highlighted in bold. Residue numbering corresponds to SEQ ID NO:65.
[0264]
[0265] Therefore, in one aspect of the present invention, a polypeptide is provided that binds to an epitope on IL-7Rα (SEQ ID NO: 65), said epitope comprising IL-7Rα selected from Glu27, Ser31, Leu57, At least one residue of Val58, Glu59, Lys77, Lys78, Phe79, Leu80, Leu81, Ile82, Thr104, Lys137, Lys138, Tyr139, Lys141, His191, Tyr192 and Phe193. Suitably, the polypeptide binds to an epitope on IL-7Rα comprising at least 8, more suitably at least 15, more suitably at least all residues of IL-7Rα selected from this list.
[0266] It can be noted that certain residues of IL-7Rα cover a particularly prominent region at the inte...
Embodiment 1
[0423] Example 1: Immunization and phage library construction
[0424] Two llamas were individually immunized with soluble human recombinant IL-7Ra. Blood was collected from two llamas at different time points during immunization and tested for IL-7Rα binding and neutralization to monitor the development of an immune response against IL-7Rα. Analysis showed that only one llama developed good anti-IL-7Rα antibody titers, while the other llama failed to respond to IL-7Rα immunization. RNA isolated from leukocytes collected from responsive llamas at the end of immunization was used to generate twelve individual phage display libraries.
Embodiment 2
[0425] Example 2: Library selection with human IL-7Rα binding activity
[0426] A library selection strategy was developed to isolate ICVDs that bind to epitopes present on the extracellular domain of the IL-7Rα subunit, including ICVDs that interfere with the binding of IL-7 to IL-7R. Several approaches were used to selectively enrich for phage displaying ICVD with IL-7Rα binding characteristics and other desirable properties, including high binding affinity and resistance to enteric proteases. E. coli was infected with phage present in the eluate from the different library selections, and individual colonies were picked into master plates and propagated to generate colony cultures. Periplasmic supernatants containing selected monoclonal ICVDs were used for primary evaluation studies to identify those with desired characteristics.
[0427] From a total of 630 library-selected colonies in 8 original master plates picked to screen, a final set of 7 primary clones were selected...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com