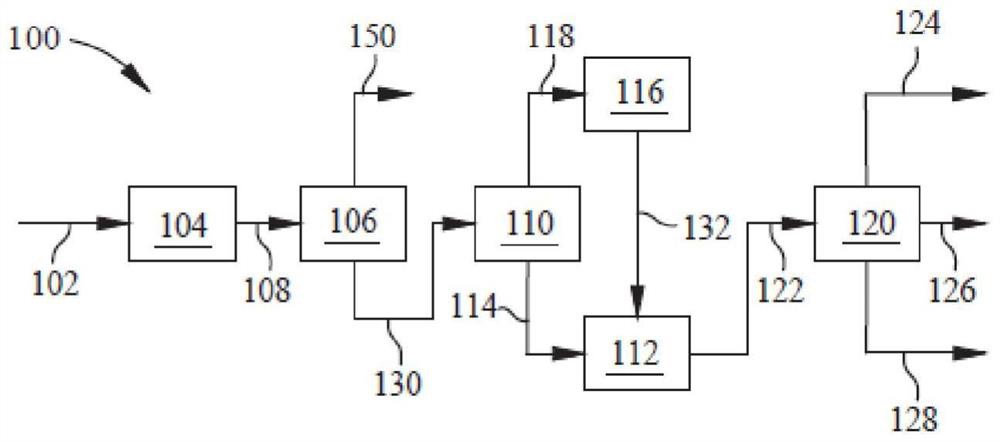
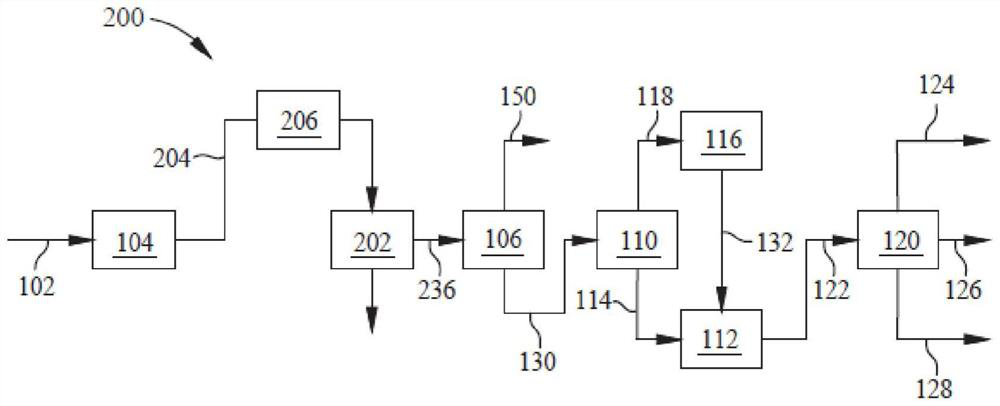
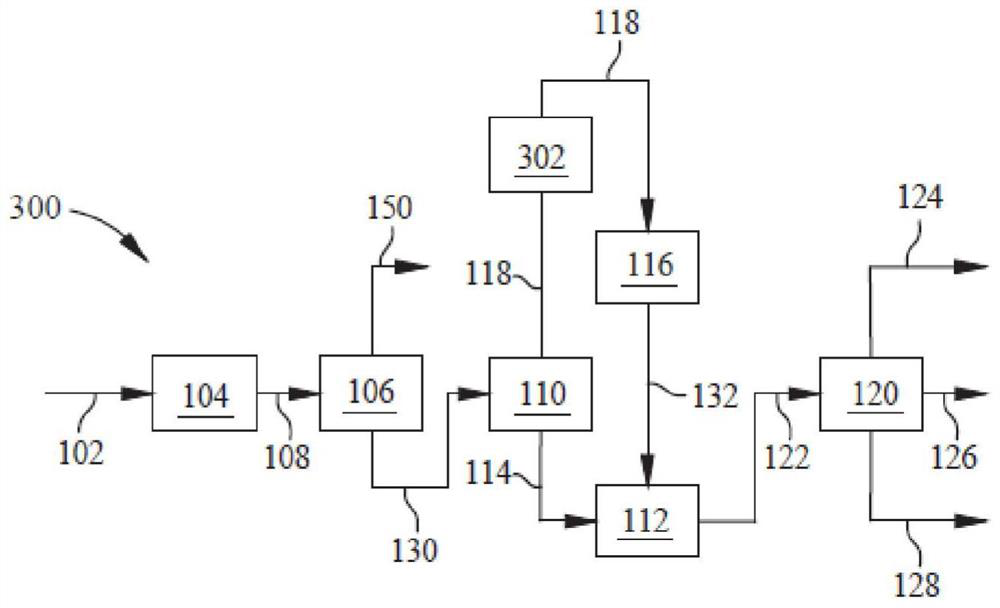
Method and device for preparing poly-alpha-olefin
A technology for olefins and olefin monomers, which is applied in the field of preparing polyalpha-olefins and devices thereof, and can solve the problems of slow output, low total product yield, low output and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0240] Example 1: Using dimethylsilylbis(4,5,6,7-tetrahydroindenyl)zirconium dimethyl, N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate and Two dimer samples were produced from the dimerization of 1-decene with tri-n-octylaluminum (TNOA) as a catalyst / activator system. The samples were then distilled without addition of bulk feed or filtration to yield >98% dimer. Initial NMR was performed to confirm the distribution of vinylidene groups to trisubstituted olefins. The samples were then heated to 270°C under nitrogen while stirring for over 22 hours and multiple samples were removed from the tank. All samples were analyzed by NMR and the resulting ratios of trisubstituted olefins to vinylidene olefins are reported in Figure 4 middle. The initial ratio of trisubstituted olefin to vinylidene olefin was about 1:1, but significant isomerization of the olefin to the trisubstituted olefin occurred within 2 hours, resulting in a 9:1 ratio.
Embodiment 2
[0241] Example 2: In the filtered sample, the cellulosic body feed was added and the slurry was filtered to remove all polar components. The sample was then distilled to yield >98% dimer. Initial NMR was performed to confirm the distribution of vinylidene groups to trisubstituted olefins. The samples were then heated to 270°C under nitrogen while stirring for over 14 hours and multiple samples were removed from the jar. All samples were analyzed by NMR. A comparison of trisubstituted dimers at similar reaction times is shown in Figure 5 middle. Figure 5 It was illustrated that dimer samples produced by bypassing the filtration resulted in significant isomerization to produce higher trisubstituted olefins. Figure 5 It was further shown that dimer samples subjected to filtration prior to isomerization showed no change in the amount of trisubstituted olefins.
Embodiment 3
[0242] Example 3: The dimer product from Example 1 was mixed with 0.7 wt% Amberlyst 15 catalyst and heated under nitrogen while stirring to 100°C for over 22 hours. NMR spectroscopy confirmed a ~1:1 distribution of vinylidene to trisubstituted vinylidene olefin. After 22 hours, the ratio by NMR was 1:29 vinylidene to trisubstituted vinylidene olefin. Filtration removes key species involved in the isomerization of vinylidene olefins to trisubstituted olefins. Reintroduction of Lewis acid species, such as Amberlyst catalyst, allows the isomerization to proceed to form predominantly trisubstituted vinylidene olefins.
[0243] In general, the present disclosure provides methods and apparatus for forming polyalpha-olefins by isomerizing a feed to form a feed having a high trisubstituted olefin dimer content. Isomerization of vinylidene dimers to trisubstituted olefin dimers provided higher overall yields of trimers. It has been found that vinylidene dimers can be isomerized in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com