Control method for improving stability of olmesartan medoxomil tablets
A technology for olmesartan medoxomil and a control method is applied in the field of control for improving the stability of olmesartan medoxomil tablets, and can solve the problem that the content uniformity of the main drug does not meet the standard, the particle size and the particle size of the auxiliary materials are greatly different, and the production efficiency of dry granulation can be solved. low problems, to avoid adverse effects, reduce moisture content, and achieve good compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0031]实施例1
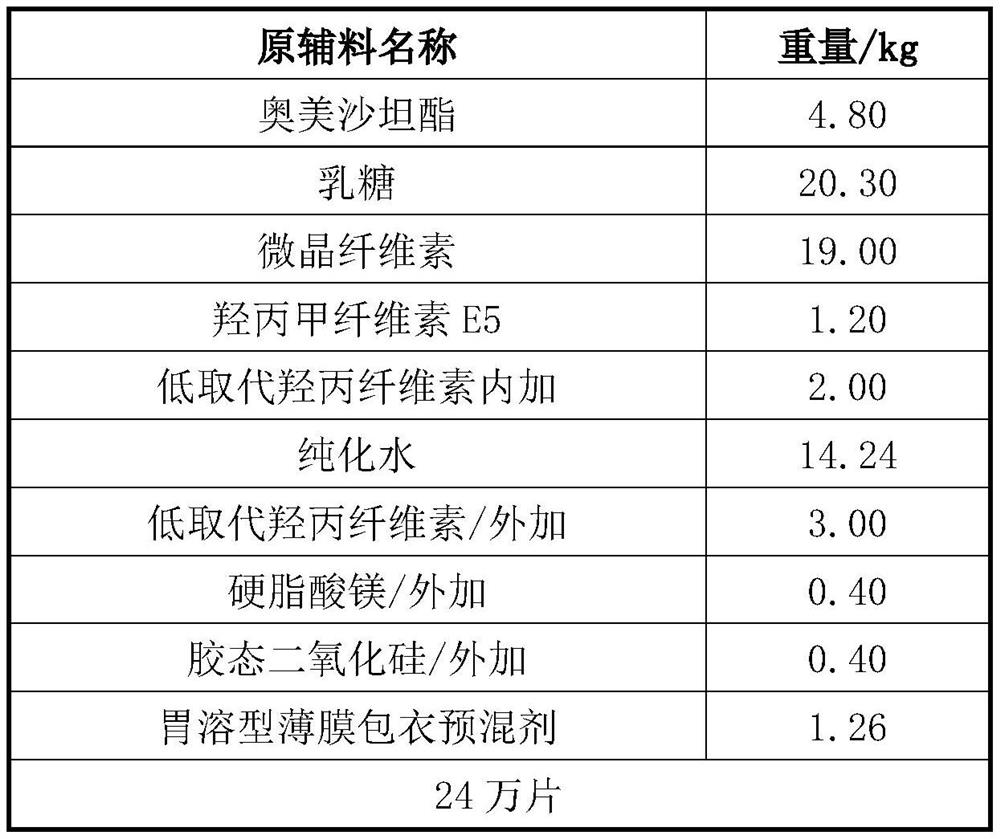
[0032]一种奥美沙坦酯片,原料如表1所示。
[0033]表1奥美沙坦酯片的原辅料成分表
[0034]
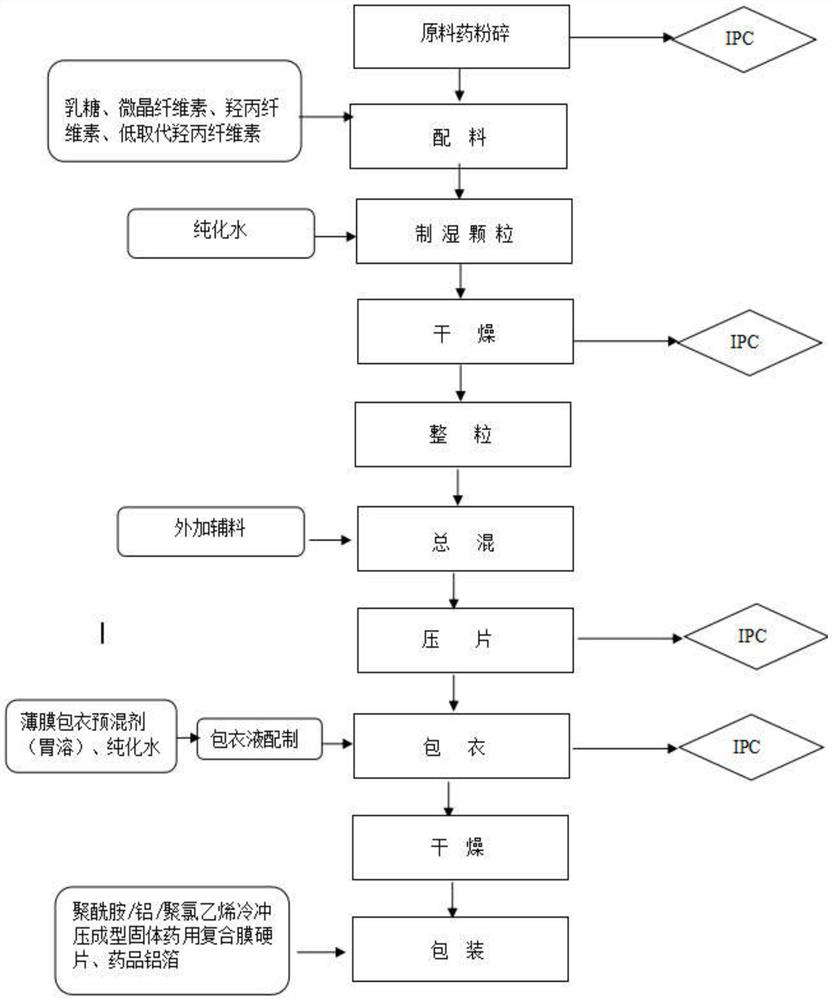
[0035]一种提高奥美沙坦酯片稳定性的控制方法,所述方法包括以下步骤:
[0036]1)奥美沙坦酯(气流粉碎)、乳糖、微晶纤维素、羟丙纤维素、低取代羟丙纤维素(内加),混合均匀,得到物料1;
[0037]2)物料1中加入纯化水,制得湿颗粒,得到物料2;
[0038]3)物料2干燥得物料3;
[0039]5)将物料3过1.0-2.0mm孔径筛网整粒,整粒后加入低取代羟丙纤维素(外加),硬脂酸镁,胶态二氧化硅,混合均匀,得到物料4;
[0040]6)将物料4压片,得物料5;
[0041]7)将物料5用水溶性胃溶型包衣粉包衣得奥美沙坦酯片,得物料6.
[0042]8)物料6干燥,控制水分在4.5%以内,双铝包装;
[0043]完成奥美沙坦酯片的制作。
[0044]优选地,所述步骤5)将物料3过1.0-2.0mm孔径筛网整粒。
[0045]优选地,奥美沙坦酯片有关物质标准杂质A不得过0.5%,奥美沙坦二聚体不得过0.5%,杂质C不得过0.3%,其他单个未知杂质(除杂质B、杂质D、杂质A、杂质C、奥美沙坦二聚体外)不得过0.2%,其它未知杂质总和不得过0.5%,总杂(除杂质B、杂质D外)不得过2.0%。
[0046]优选地,所述步骤2),干燥后颗粒干燥失重1.5%以内,检测方法卤素水分测定仪,105℃,10min。
[0047]优选地,所述步骤8)将物料6用热封循环烘箱60±5℃条件下干燥60min,控制水分在4.5%以内。
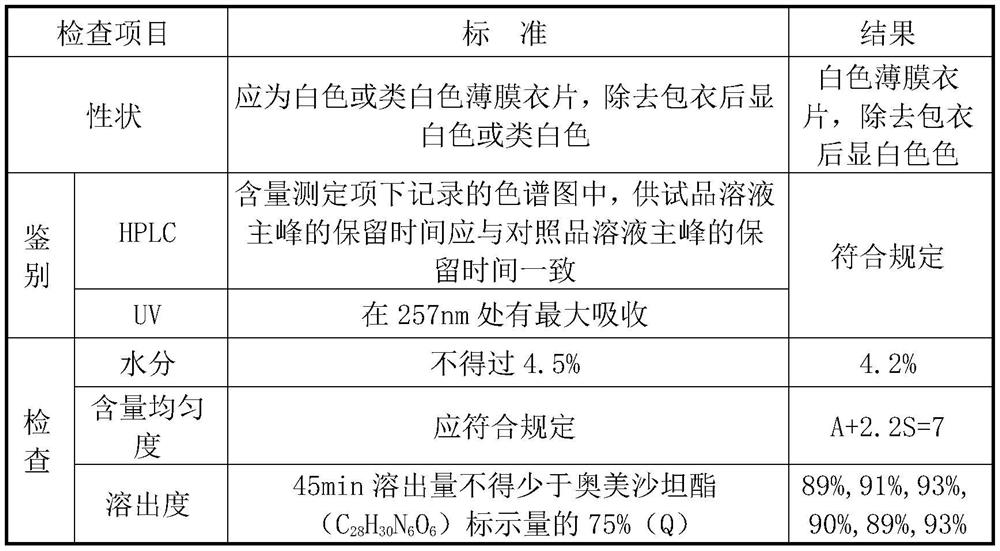
[0048]成品检测结果如表2。
[0049]表2
[0050]
[0051]
[0052]通过实验制备实施例1样品进行加速条件(40℃±2℃ / 75%RH±5%RH)下稳定性试验,杂质A考察结果如表3。
[0053]表3
[0054]杂质A含量 / %水分0天加3月加6月实施例1产品4.2%未检出(<0.1%)0.23%0.45%
Example Embodiment
[0055]实施例2
[0056]以实施例1为基础,改变制剂组分。其他同实施例1。
[0057]所述奥美沙坦酯片的原料包括按照重量份计的以下组分,批量24万片:
[0058]表4不同组分制备的奥美沙坦酯片
[0059]
[0060]
[0061]杂质A加速稳定考察结果如表4。
[0062]表5
[0063]杂质A含量 / %水分0天加3天加6天实施例1产品4.2%未检出0.23%0.45%实施例2-1产品4.3%未检出0.36%0.70%实施例2-2产品4.2%未检出0.39%0.75%实施例2-3产品4.5%未检出0.42%0.80%实施例2-4产品4.7%未检出0.45%0.81%
[0064]由表5可知,如果处方中加入易吸湿物料,如交联聚维酮和聚维酮K30,或者水分含量较高,如玉米淀粉和微晶纤维素,将不利于奥美沙坦酯片杂质A控制。
Example Embodiment
[0065]实施例3
[0066]以实施例1为基础,改变步骤3)控制条件。
[0067]实施例1:所述步骤3)将物料3的水分含量控制在1.2%。
[0068]实施例3-1:所述步骤3)将物料3的水分含量控制在1.5%。
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com