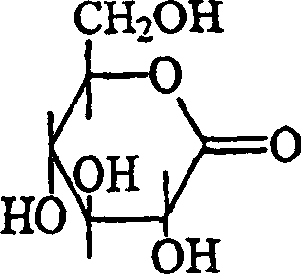
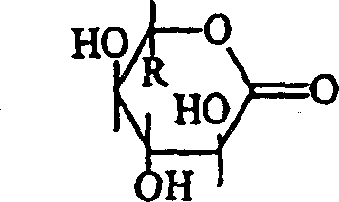
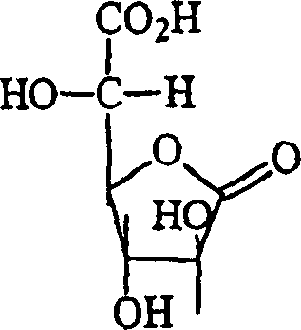
Anti-irritants in cosmetic compositions
A technology of composition and cosmetics, applied in the direction of cosmetics, cosmetic preparations, drug combinations, etc., can solve the problems of not teaching gluconolactone anti-irritants, etc., and achieve the effect of reducing skin irritation and improving irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The possible anti-inflammatory effects of test compounds were assessed by their ability to inhibit IL-la-induced PGE2. IL-1α and PGE2 are well known mediators of skin inflammation. See Greaves et al., "Prostaglandins, leukotrienes, phospholipases, platelet-activating factor, and cytokinesis: A comprehensive approach to the treatment of human skin inflammation", Arch. Dermatol. Res. (1988), 280 [Supp]: S33-S41 .
[0066] Neonatal human dermal fibroblasts (paragraphs 5-9) were seeded at a density of 7500 cells per well on tissue culture treated plates (Corning-Costar, Corning, NY) in 96 wells. The medium used was Dulbecco's modified Eagle's medium (DMEM), high glucose (Gibco / Biotechnology, Gaithersburg, MD) supplemented with 2 mM L-glutamine, 10% fetal bovine serum and antibiotic and antifungal solutions (also both See Biotechnology). After 48 hours, each well was rinsed twice with 200 microliters of serum-free DMEM and the wells were dosed with 200 microliters of DMEM...
Embodiment 2
[0077] Stimulus test method
[0078] Four-exposure patch test: The objective is to compare the degree of irritation caused by repeated patch application of each test substance, keeping the test substance in contact with the skin under closed conditions. The panelist's upper outer arm was designated as the area of application. A patch (25 mm Hill Top Chamber filled with 18 mm diameter Webril filler discs) was placed in place using a bandage-type dressing (Scanpor tape). Use both upper arms of the team member. Patches were applied in a balanced random order.
[0079]Patches were applied at 9:00 am on Monday and removed at 9:00 am on Tuesday (24 hour exposure). A new set of patches was applied Tuesday at 3:00 pm and removed Wednesday at 9:00 am (18 hours exposure). A third set of patches was applied on Wednesday at 3:00 pm and removed on Thursday at 9:00 am (18 hours exposure). The final set of patches was applied on Thursday at 3:00 pm and removed on Friday at 9:00 am (1...
Embodiment 3
[0101] Compositions containing the components shown in Table 4 were tested using this challenge test method. 20 subjects were tested. The results obtained are summarized in Table 4. The higher the grade sum, the lower the stimulation.
[0102] Table 4
[0103] combination
Component
Stimulus Score (4 days)
1
basic formula
68.5 a
2
Basic Formula (Composition #1) + 8% Ethanol
Acid, pH3.8
57
6
Basic Formula (Composition #1) + 8% Gluconic Acid
100.5 a
[0104] a: Significantly lower irritation than composition 2 (2 days).
[0105] b: significantly lower than compositions 1 and 2 in irritation.
[0106] From the results in Table 4, it can be seen that gluconolactone (composition #6) significantly reduced the irritation of the base formulation which did not contain glycolic acid. This example demonstrates that the method of the present invention can be used to reduce skin irritatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com