Method for preventing burning explosion of low-carbon hydrocarbon and application thereof
A low-carbon hydrocarbon and reaction gas technology, which is applied to chemical instruments and methods, purification/separation of hydrocarbons, explosive materials, etc., can solve the problems of not effectively prolonging the induction time of spontaneous combustion to prevent explosion, personal injury, equipment damage, etc., to achieve Prolong the induction time of spontaneous combustion, avoid equipment damage, and avoid the effect of explosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
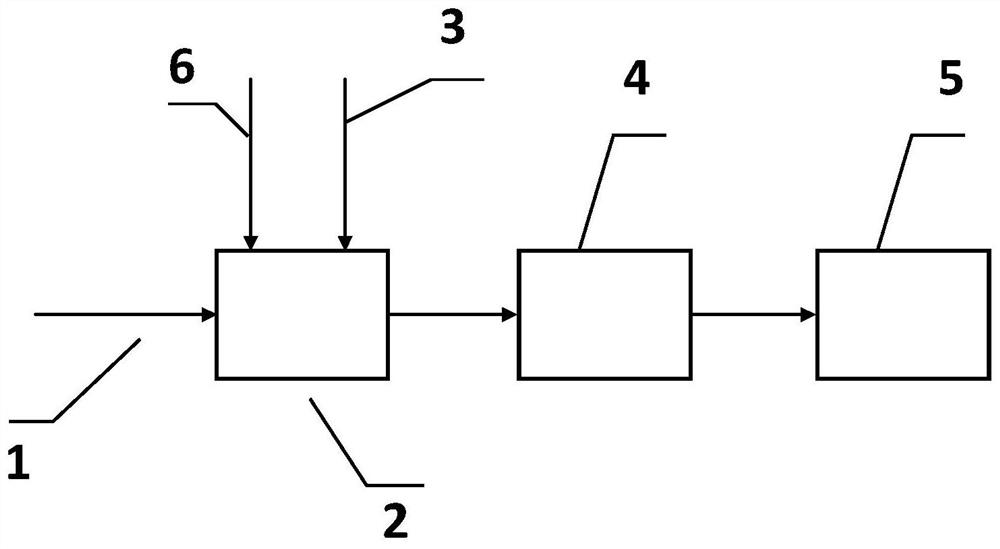
[0050] First, methane enters the buffer tank through the feed line, and oxygen enters the buffer tank through the feed line. The molar ratio of oxygen and methane is 1:2. After the temperature rises to about 500 °C in the buffer tank, it passes through the column. The plug pump is driven into the gas explosion test device. According to the heating rate of 10°C / min, the temperature of the explosion test device is raised to 800°C and the pressure is 1 bar. Through the gas explosion test device, 1 bar is obtained. At 800°C, the methane The self-ignition induction time of the gas is T 0 , and then flush N into the flash tank through the gas distribution feed line 2 , according to the amount of nitrogen flushed, the volume concentration of methane is diluted to 0.9, 0.8, 0.7, 0.6, 0.5, and then a series of concentration gradients of methane and N 2 The mixed gas was passed into the gas explosion test device, and the spontaneous combustion induction time T of methane at different v...
Embodiment 2
[0060] The natural induction time test of low-carbon hydrocarbons was carried out according to the method of Example 1, except that methane was replaced by ethane.
[0061] test experiment
[0062] In the mixture of ethane, oxygen and nitrogen, the volume concentration of ethane is 0.15, and the result obtained by fitting the above method at 800 °C and a pressure of 1 bar is the sum of the self-ignition induction time T of ethane and the concentration of ethane. The relationship equation between: T=a 1 m x T 0 +b 1 mT 0 +c 1 , the self-ignition induction time of ethane in the mixture is 130ms, and then, under the condition of 800 ° C and 1 bar, the volume concentration of ethane in the mixture of ethane, oxygen and nitrogen is controlled to be 0.15, at this time, x is 2.15. The test found that no explosion occurred when the oxidation reaction time was 110ms, 115ms, 120ms and 125ms, and when the oxidation reaction time was controlled to 130ms, explosion occurred.
Embodiment 3
[0064] The natural induction time test of low-carbon hydrocarbons was carried out according to the method of Example 1, except that methane was replaced by ethylene.
[0065] test experiment
[0066] In the mixture of ethylene, oxygen and nitrogen, the volume concentration of ethylene is 0.18, and the relationship equation between the self-ignition induction time T of ethylene and the concentration of ethylene at 800 °C and a pressure of 1 bar obtained by fitting the above method : T=a 1 m x T 0 +b 1 mT 0 +c 1 , the self-ignition induction time of ethylene in the mixture is 120ms, and then, at 800 ° C and the pressure of 1 bar, the volume concentration of ethylene in the mixture of ethylene, oxygen and nitrogen is controlled to be 0.18, at this time, x is 2.52 , the test found that when the oxidation reaction time was 100ms, 105ms, 110ms and 115ms, no explosion occurred, and when the oxidation reaction time was controlled to 120ms, explosion occurred.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com