Methods for treating neurodegenerative disorders
A technology for disorders and neurasthenia, applied in neurological diseases, biochemical equipment and methods, pharmaceutical formulations, etc., can solve problems such as uncertain pathogenic processes and effort limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
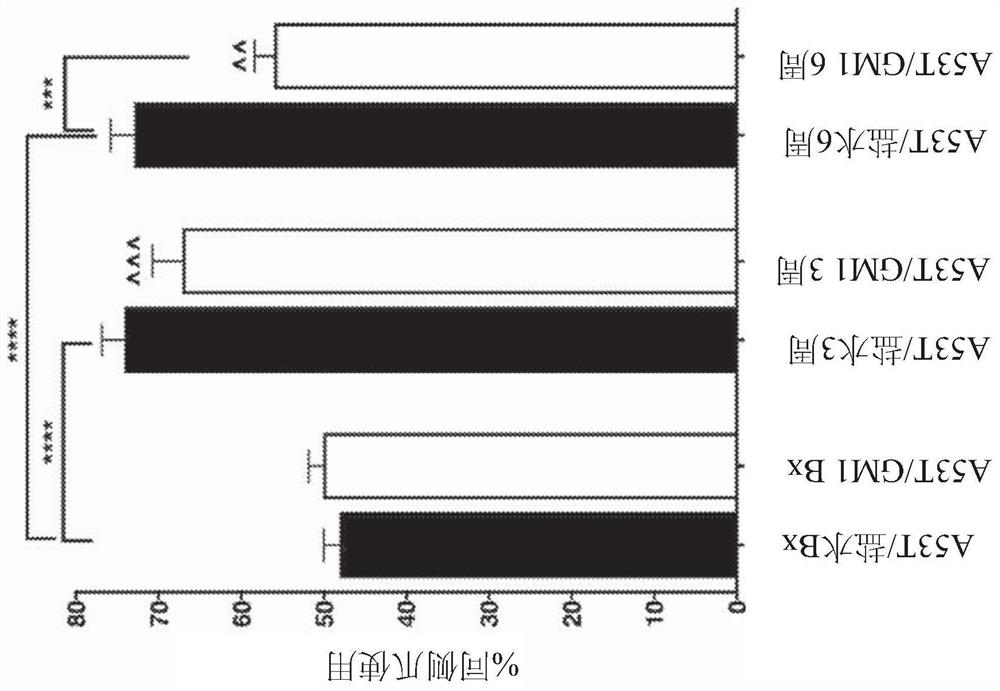
[0165] Example 1 - Early onset GM1 administration partially protects motor behavior and striatal DA levels
[0166] Spontaneous forelimb use was assessed in AAV-A53Tα-synuclein-transduced animals using the cylinder test, and treatment had a significant main effect, with protection observed in GM1-treated animals (F(5,102)=16.59, P Figure 1A ). In animals that received GM1 administration starting 24 hours after AAV-A53Tα-synuclein injection, limb use asymmetry (percentage of ipsilateral limb use) also increased at 3 and 6 weeks after AAV-A53Tα-synuclein injection increased, but this increase was only significantly different from baseline at 3 weeks (mean ± SEM: baseline: 50.1 ± 1.8%; 3 weeks: 67.1 ± 3.7%, 6 weeks: 56.0 ± 2.5%) ( Figure 1A ). GM1-treated animals had significantly less asymmetric limb use at 6 weeks compared to saline-treated animals at 6 weeks (P Figure 1A ). Animals receiving AAV blank vector injections showed no significant changes in limb use (% ipsilate...
Embodiment 2
[0172] Example 2 - Early initiation GM1 administration partially protects SN neurons from alpha-synuclein-induced toxicity
[0173] To investigate the potential protective role of GM1 in the context of PD-related pathology, the extent to which GM1 administration affects the survival of A53Tα-synuclein-overexpressing substantia nigra DA neurons was examined. Significant reduction in the number of TH+ cells in the SNc on the ipsilateral injected side: AAV-A53T-α-synuclein injection resulted in a 60.0±2.3% loss of TH+ neurons compared to the contra (non-injected) side ( Figures 3A-3B ). Animals receiving early initiation GM1 administration had 43.7±2.7% loss of TH+ neurons compared to the non-injected side (t(28)=4.379, P=0.0002) ( Figures 3A-3B ;Table 2). Similarly, the number of cresyl violet-stained cells in the SNc was significantly affected by AAV-A53T-α-synuclein injection and GM1 treatment. AAV-A53T-α-synuclein injection resulted in a 54.9±1.8% loss of cresyl violet...
Embodiment 3
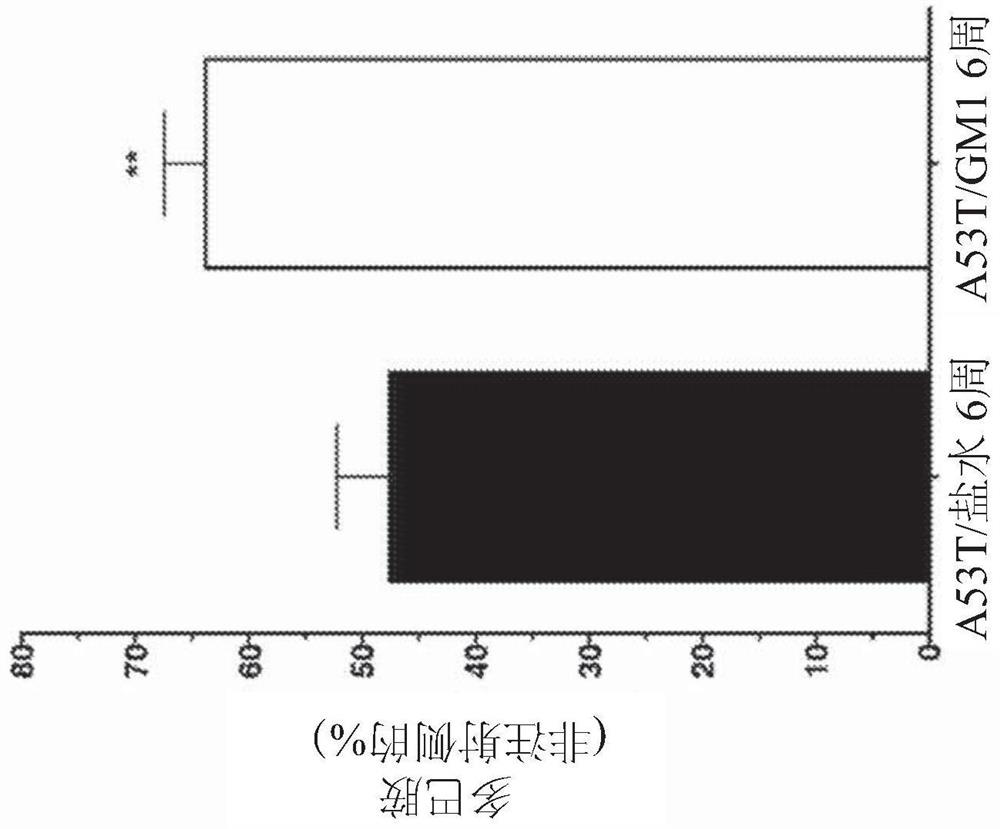
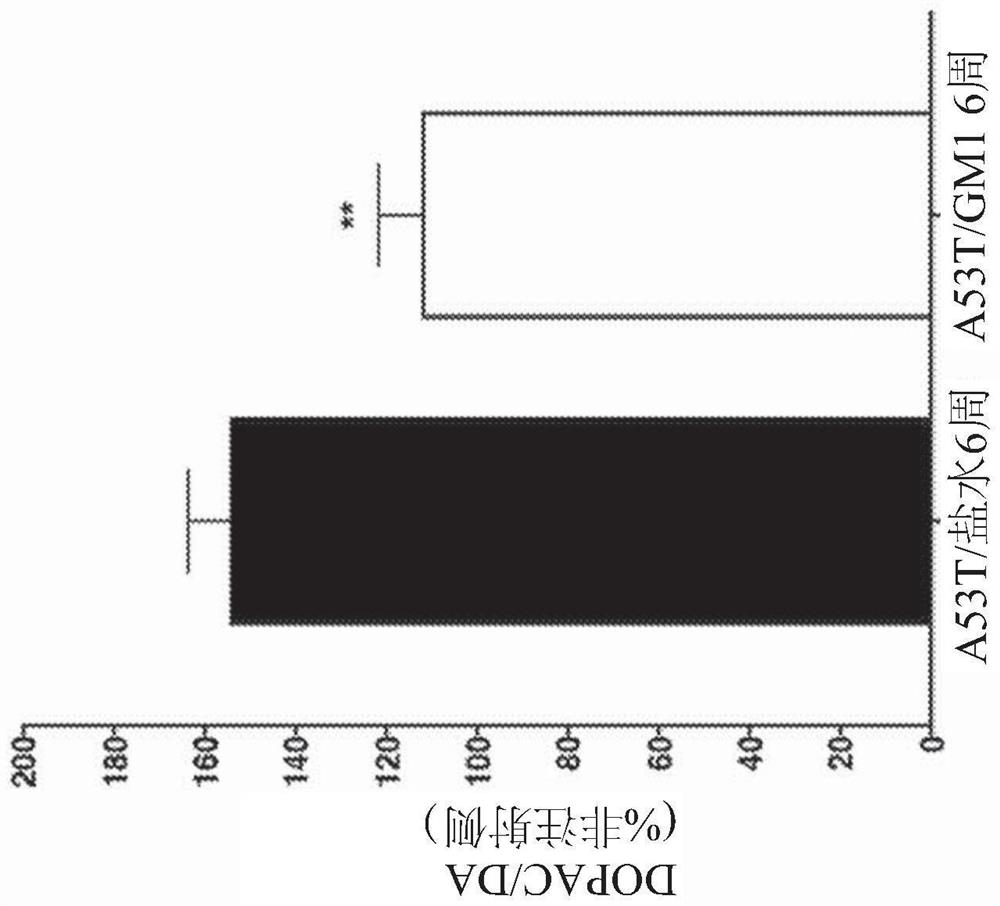
[0177] Example 3 - Delayed onset GM1 administration partially restores motor behavior and preserves striatal DA levels
[0178] Animals that received AAV-A53Tα-synuclein followed by saline for 8 weeks also developed significant asymmetry in paw use, preferentially using the forepaw on the ipsilateral side of the virus injection to contact the cylinder ( Figure 4A ). When tested 3 weeks after AAV-A53Tα-synuclein injection, saline-treated animals showed a significant increase in the percentage of ipsilateral limb use, similar to that seen in the aforementioned animals, and was consistently observed at 8 weeks after virus injection to (mean ± SEM; baseline: 50.9 ± 2.4%; 3 weeks: 74.5 ± 3.8%, 8 weeks: 81.4 ± 4.5%) ( Figure 4A ). There was a significant treatment effect supporting the GM1 treatment group (F(5,84)=19.14, P Figure 4B ).
[0179] Eight weeks after AAV-A53Tα-synuclein injection, striatal DA levels on the virus-injected side of the brains of saline-treated animals ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com