Novel conjugation chemistry for catalytic antibodies 38C2
An antibody and conjugation technology, applied in the direction of antibodies, chemical instruments and methods, drug combinations, etc., can solve problems such as unmet needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1 Computer simulated Lys99 arylation
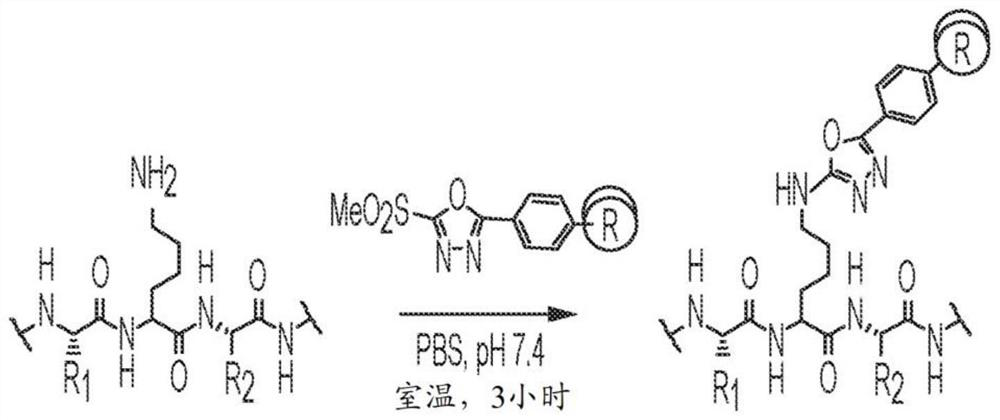
[0087] The nucleophilicity of the ε-amino group of Lys99 of h38C2 prompted us to investigate alternative irreversible covalent conjugation chemistries that could further increase the accessible payload space. Due to the hydrophobic nature of the Lys99 microenvironment, we hypothesized that Lys arylation, which has not yet been reported for antibody conjugation, could provide a suitable pathway. Specifically, we purposely tested heteroarylmethylsulfonyl compounds as serum-stable alternatives to maleimide-based conjugation to antibodies with engineered free cysteine residues. Our study focused on methyl sulfone phenyl oxadiazole (MS-PODA) ( Figure 1A ).
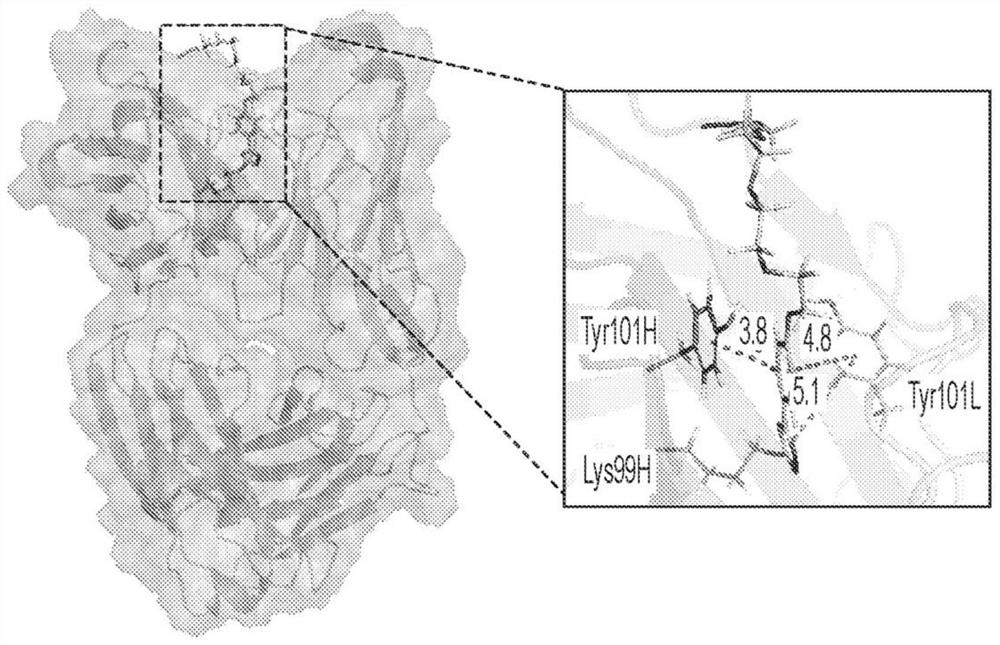
[0088] Based on the proposed reaction of MS-PODA with the ε-amino group of Lys99 ( Figure 1A ), we used computational modeling to dock compounds into the hydrophobic pocket of h38C2. This is based on the recently resolved crystal structure of the h38C2 Fab (PDBID 6...
Embodiment 2
[0089] Example 2 Probing Lys99 Arylation with MS-PODA Derivatives of Fluorescein
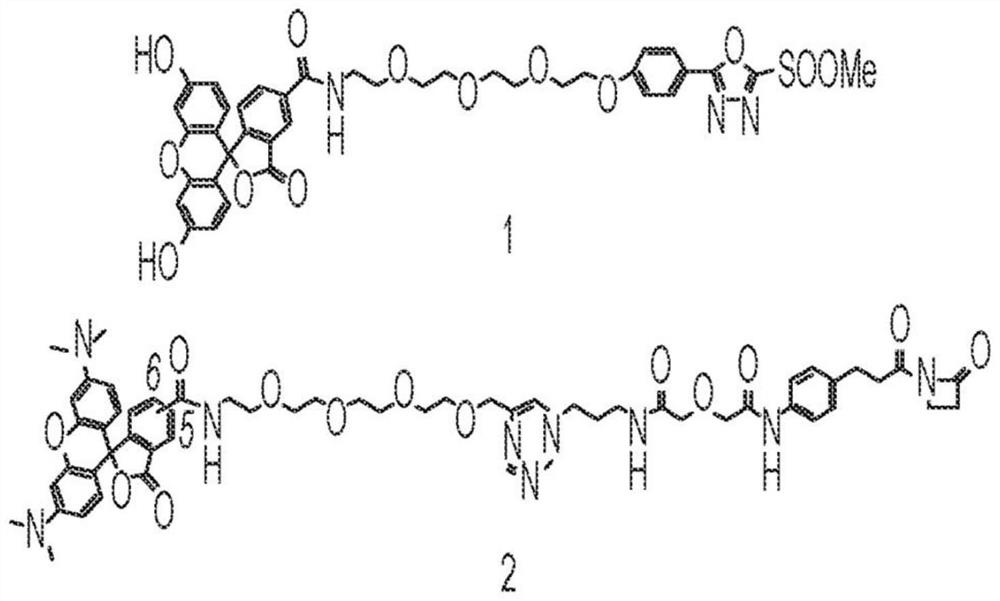
[0090] This example describes the detection of efficient, selective and stable arylation of Lys99 with MS-PODA derivatives of fluorescein. To probe covalent conjugation to Lys99 in vitro, we used the previously described MS-PODA derivative of fluorescein (Compound 1; Figure 2A ). For comparison, we included the previously described beta-lactam hapten derivative of tetramethylrhodamine (TAMRA) (Compound 2; Figure 2A ). To pinpoint conjugation on Lys99, we also cloned, expressed and purified h38C2 IgG1 with Lys99Ala mutation. Unpurified separated by reducing and non-reducing SDS-PAGE after incubation of h38C2 and h38C2_Lys99Ala IgG1 with a 5-fold molar excess (5 equiv per reactive Lys residue) of compounds 1 and 2 in PBS for 4 hours at room temperature The antibody conjugated with unconjugated antibody was analyzed by Coomassie blue staining and in-gel fluorescence ( Figure 2B ). This a...
Embodiment 3
[0092] Example 3 MS-PODA-mediated chemical programming
[0093] The efficient, selective and stable conjugation of fluorescein derivatives of MS-PODA to Lys99 of h38C2 prompted us to investigate MS-PODA conjugation in the context of the known therapeutic utility of h38C2, including chemical programming. 8 To confer high specificity and affinity to h38C2 for the small molecule binding sites of two different cell surface receptors, we synthesized a β-lactam hapten derivative of folic acid and an MS-PODA derivative of folic acid (compound 3, respectively and 5; Figure 4A ) and the β-lactam hapten derivative of LLP2A and the MS-PODA derivative of folic acid (compounds 4 and 6, respectively; Figure 4A ). It should be noted that the synthetic route to MS-PODA-containing structures is more straightforward compared to β-lactam functionalized ligands. The preparation of MS-PODA moieties can be achieved with readily available commercial reagents, and the incorporation of ligands ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com