Therapeutic agent for non-alcoholic fatty liver disease
A tablet, benzoxazole technology, applied in the field of non-alcoholic fatty liver disease prevention and/or therapeutic agent, can solve the problems such as unestablished recommended treatment methods, achieve the effect of inhibiting fat deposition and reducing Kupffer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
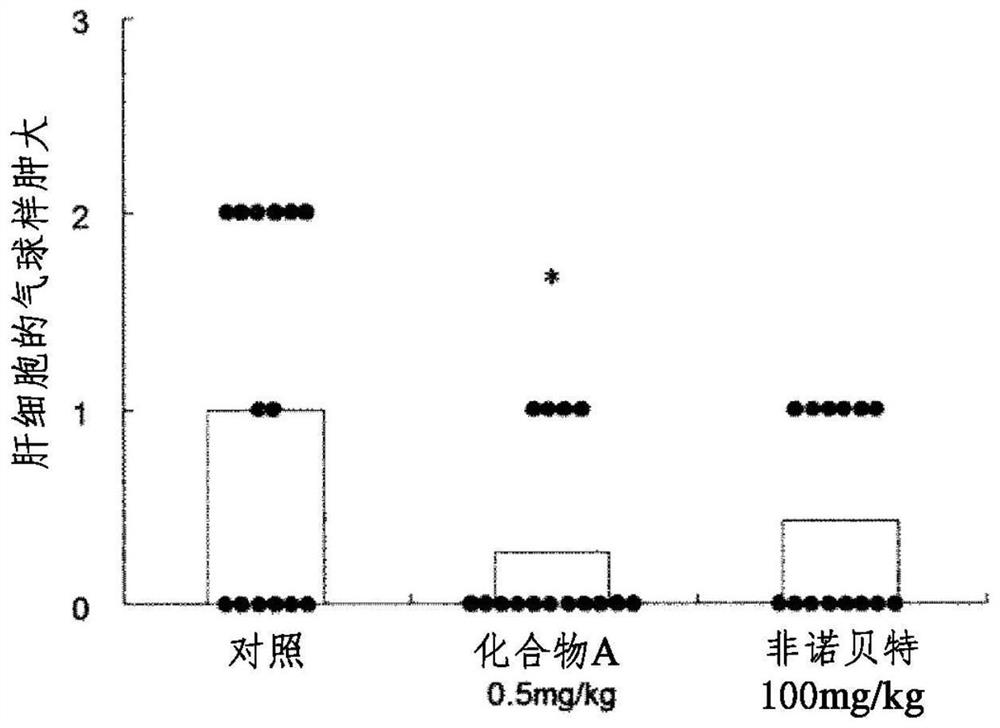
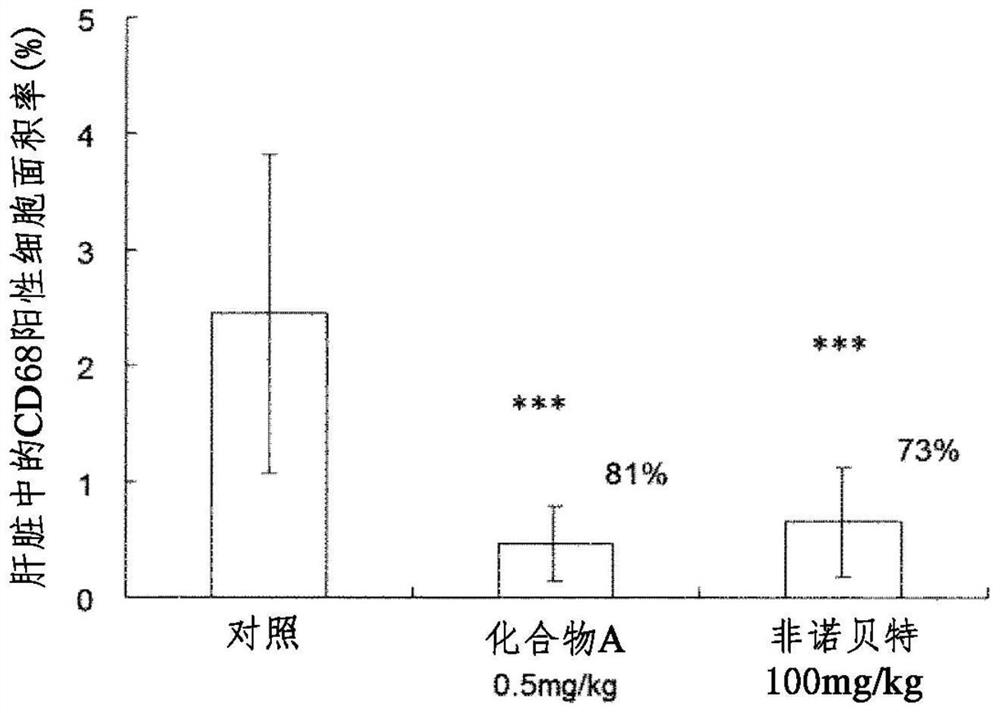
[0064] The role of Example 1 in the LDL receptor knockout mouse model of NASH
[0065] The role in high-fat / high-cholesterol diet (Western diet)-loaded LDL receptor knockout mice (Non-Patent Document 4) known to have fatty liver and hepatitis development, which is characteristic of NASH, was investigated.
[0066] In addition, in this test, (R)-2-{3-{[N-(benzoxazol-2-yl)-N-3-(4 disclosed in Example 85 of Patent Document 1 above was used. -Methoxyphenoxy)propyl]aminomethyl}phenoxy}butyric acid (compound A).
[0067] 1) Using animals:
[0068] Male LDL receptor knockout mice (B6.129S7-Ldlr / J, Jackson Laboratories). NASH was developed by ad libitum intake of Teklad Custom Research Diet (Harlan Teklad Company, TD. 88137) as a high fat / high cholesterol diet (Western diet) during 13 weeks from about 8 weeks of age.
[0069] 2) Group composition:
[0070] Mice were loaded with a high-fat / high-cholesterol diet (Western diet), and blood collection and body weight measurements...
Embodiment 2
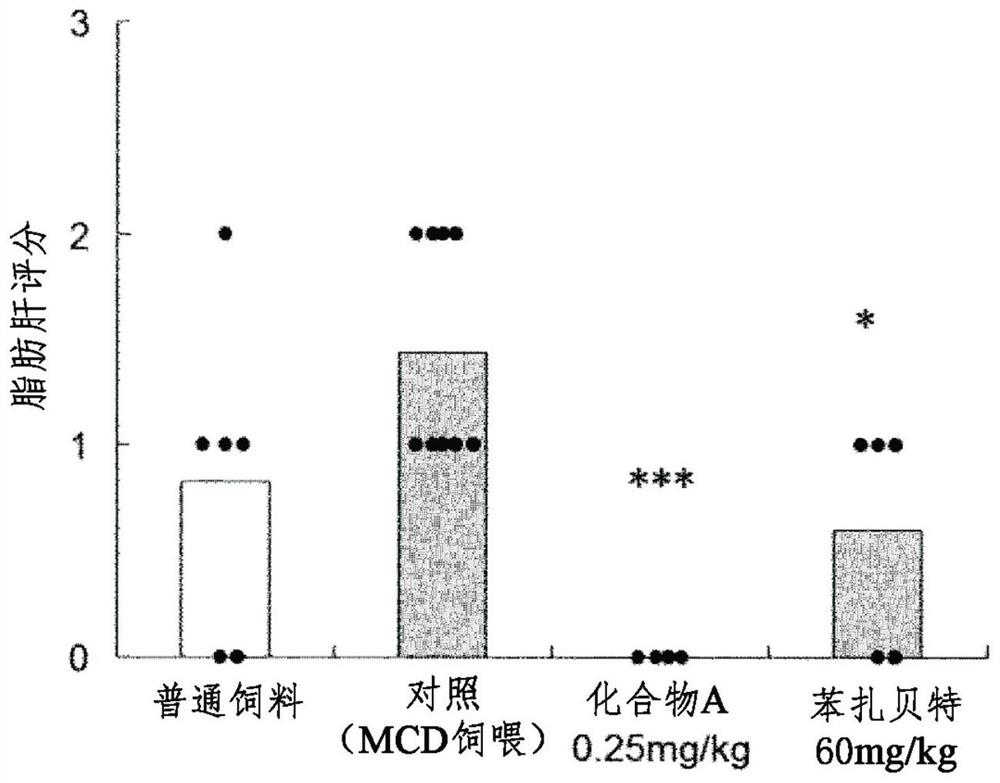
[0082] Example 2 KK-A in the use of methionine / choline deficient dietary loads y Role in a mouse model of NASH
[0083] Investigated KK-A of MCD diet known to cause the onset of fatty liver disease characteristic of NASH by loading experimental animals with a methionine / choline deficient diet (MCD diet) y Effects in mice (Nakano S. et al., Hepatol Res., 38(10), 1026-39, 2008). In addition, in this test, as in Example 1, (R)-2-{3-{[N-(benzoxazol-2-yl)-N-3 disclosed in Example 85 of Patent Document 1 above was used -(4-Methoxyphenoxy)propyl]aminomethyl}phenoxy}butyric acid (compound A).
[0084] 1) Using animals:
[0085] Use male KK-A in experiments y mouse (KK-A y / TaJcl, Japan クレア Co., Ltd.). Mice were made to develop NASH by ad libitum MCD diet for 16 weeks starting at about 12 weeks of age.
[0086] 2) Group composition:
[0087] The groups were divided into the following groups: normal diet group, control group (MCD dietary load), compound A 0.25 mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com