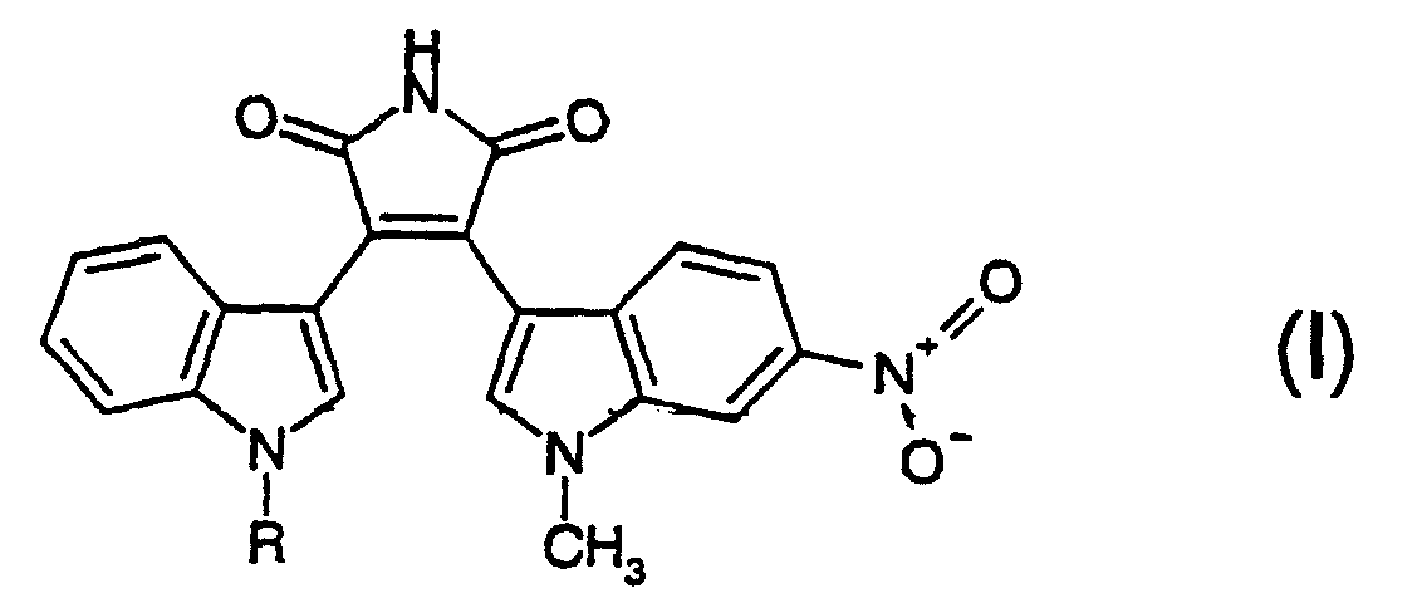
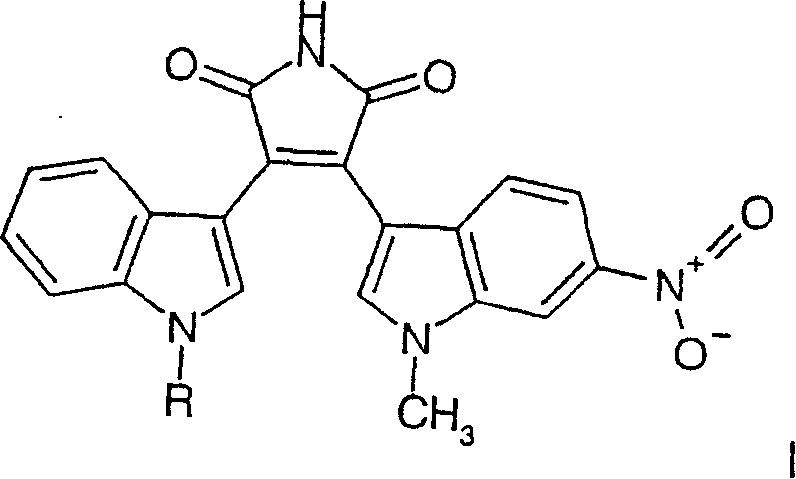
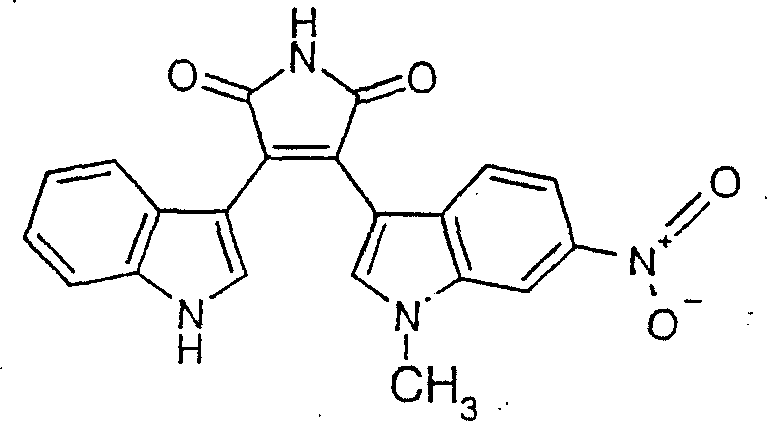
Substituted bisindolylmaleimides for inhibition of cell proliferation
A technology of indole and alkyl, applied in the field of substituted pyrrole compounds, which can solve the problems of poor solubility, unsuitability, inconvenient therapeutic application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: 3-[2-(2-Methoxy-ethoxy)-ethoxy]-propionic acid 3-[4-(1-methyl-6-nitro-1H-indole-3 -yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-indol-1-ylmethyl ester
[0100] 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide·HCl ("EDC·HCL"; Aldrich, 77 mg, 0.40 mmol) in dry THF (12 ml) at 22°C The suspension in DMAP (Aldrich) (55 mg, 0.45 mmol) was treated for 2 min. To this was added 3-(1-hydroxymethyl-1H-indol-3-yl)-4-(1-methyl-6-nitro-1H-indol-3-yl)-pyrrole-2,5 - Diketone (110 mg, 0.26 mmol) (prepared below). The mixture was stirred for 20 minutes, to which was added 2-(2-methoxy-ethoxy)-ethoxy]-propionic acid (CAS: 209542-49-4) (120 mg, 0.62 mmol). Stirring was continued for 4 hours at 22°C. All solvents were evaporated and the product was purified by silica gel chromatography to give 130 mg of 3-[2-(2-methoxy-ethoxy)-ethoxy]-propionic acid 3-[4-(1-methyl-6-nitroso yl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-indol-1-ylmethyl ester. (80% yield)
[0101] 3-(1-...
Embodiment 2
[0102] Example 2: O-[2-[[2,5-dihydro-3-[4-(1-methyl-6-nitro-1H-indol-3-yl)-2,5-dioxo Sub-2,5-dihydro-pyrrol-3-yl]-indol-1-yl]methoxycarbonyl]ethyl]-O'-methylpolyethylene glycol 2000
[0103] To 3-(1-hydroxymethyl-1H-indol-3-yl)-4-(1-methyl-6-nitro-1H-indol-3-yl)-pyrrole at -78°C -2,5-Dione (200 mg, 0.5 mmol) (prepared as described in Example 1 above) in dichloromethane was added triethylamine (0.6 mmol) followed by O-(2-carboxyethyl) -O'-Methyl polyethylene glycol 2000 acid chloride (0.6 mmol) (prepared according to standard procedures for mono-methyl polyethylene glycol 2000 propionic acid). The solution was stirred at room temperature for 3 hours and the solvent was evaporated. The residue was purified by silica gel flash chromatography to give 1 g of O-[2-[[2,5-dihydro-3-[4-(1-methyl-6-nitro-1h-indol-3-yl)-2 , 5-dioxo-2,5-dihydropyrrol-3-yl]-indol-1-yl]methoxycarbonyl]ethyl]-O'-methyl polyethylene glycol 2000 (yield 80 %).
Embodiment 3
[0104] Example 3: The following compounds were prepared in the same manner as in Example 2:
[0105] a) 2,3-Dimethoxy-benzoic acid 3-[4-(1-methyl-6-nitro-1H-indol-3-yl)-2,5-dioxo-2,5 -Dihydro-1H-pyrrol-3-yl]-indol-1-ylmethyl ester;
[0106] b) 3-Diethylaminomethyl-benzoic acid 3-[4-(1-methyl-6-nitro-1H-indol-3-yl)-2,5-dioxo-2,5 -Dihydro-1H-pyrrol-3-yl]-indol-1-yl methyl ester hydrochloride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com