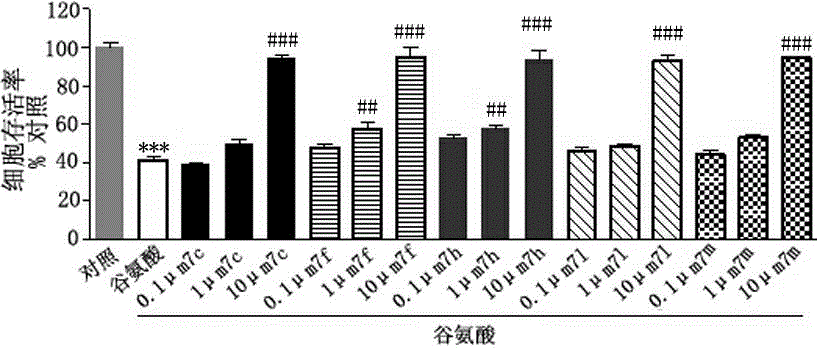
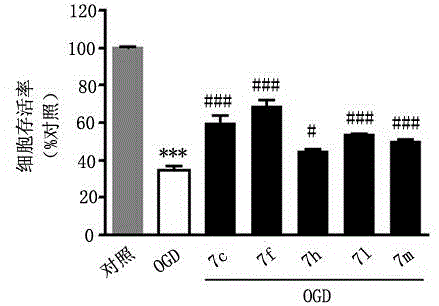
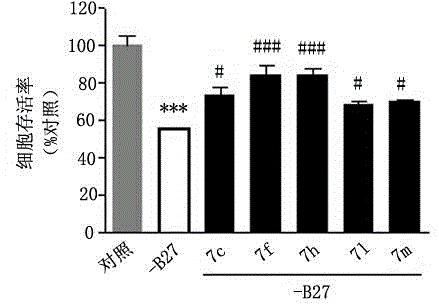
3-(1,2,4-triazolo(4,3-a)pyridine-3-yl)-4-(1H-indole-3-yl)maleimide derivative and preparation method and application thereof
A technology of maleimide and triazole, applied in drug combination, cardiovascular system diseases, organic chemistry, etc., can solve problems such as narrow time window, easy to cause bleeding, etc., achieve low equipment requirements and good product yield , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: 2-(1,2,4-triazolo[4,3- a Preparation of ]-3-yl)ethyl acetate (2)
[0032] Add 3.0 g (45.8 mmol) 2-hydrazinopyridine, 22.0 g (137.5 mmol) diethyl malonate and 60 mL phosphorus oxychloride into the three-necked flask, and reflux for 3 h. After the reaction, the reaction liquid is cooled. Pour into 300 mL of ice water, neutralize to weak alkalinity with sodium bicarbonate, fully extract with ethyl acetate, combine organic phases, dry over anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure, and use silica gel column chromatography (petroleum ether) for the residue : Ethyl acetate=2:1) to obtain 2.7g white solid 2 , yield 48%, melting point: 113-115 o c. 1 H NMR (500 MHz, DMSO- d 6 ) δ 8.45 (d, J = 7.0 Hz, 1H), 7.77 (d, J = 9.2 Hz, 1H), 7.48-7.30 (m, 1H), 7.01 (t, J = 6.8 Hz, 1H), 4.42 (s, 2H), 4.13 (q, J = 7.1 Hz, 2H), 1.19 (t, J = 7.1 Hz, 3H).
Embodiment 2
[0033] Example 2: 2-(1,2,4-triazolo[4,3- a Preparation of ]-3-yl)acetamide (3)
[0034] Add 2.0 g (9.8 mmol) to the pressure reaction vessel 2 , 30 mL ammonia saturated solution in methanol, 90 o C reacted for 6 h. The reaction liquid was cooled and filtered, and the filter cake was washed with a small amount of methanol and dried to obtain 0.88 g of white solid 3 , the yield is 51.3%, melting point: 247-249 o c. 1 H NMR (500 MHz, DMSO- d 6 ) δ 8.39 (d, J = 7.0 Hz, 1H), 7.81 (s, 1H), 7.73 (d, J = 9.2 Hz, 1H), 7.39-7.31 (m, 1H), 7.24 (s, 1H), 6.96 (t, J = 6.8 Hz, 1H), 4.13 (s, 2H). 13 C NMR (125 MHz, DMSO- d 6 ) δ168.76, 149.31, 142.61, 127.22, 124.42, 114.96, 112.80, 31.47. ESI-MS: m / z [M+H] + 177. A nal. Calcd for C 8 h 8 N 4 o 2 : C, 54.54; H, 4.58; N, 31.80. Found: C, 54.66; H, 4.31; N, 31.64.
Embodiment 3
[0035] Example 3: Preparation of 2-(1H-indol-3-yl)-2-oxoacetic acid methyl ester (5a)
[0036] Add 3.0 g (0.026 mol) of indole and 30 mL of anhydrous ether into a three-neck flask, stir to dissolve, and control the temperature from 0 to 5 o C, Slowly add 3.4 g (0.026 mol) of oxalyl chloride in anhydrous ether (5 mL) solution, keep it warm for 1 h after the drop, then cool down to about -25 ℃, add dropwise 16.3 g of sodium methoxide in methanol solution (17.5 %, 0.052 mol), kept stirring for 30 min after dropping, poured the reaction solution into 100 mL of ice water, filtered it with suction, washed with water (3 × 10 mL), washed with dichloromethane (2 × 10 mL), and dried to obtain 4.5 g light yellow solid 5a , the yield is 86.3%, melting point: 208-210 o c. 1 H NMR (500 MHz, DMSO- d 6 ) δ 12.48 (brs, 1H), 8.46 (d, J = 3.5 Hz, 1H), 8.16 (d, J = 7.0 Hz, 1H), 7.55 (d, J = 7.0 Hz, 1H), 7.32-7.26 (m, 2H), 3.90 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com