Aspartic acid lomefloxacin powder and preparing method thereof
A lomefloxacin aspartate and powder technology, which is applied in powder delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as lax drawing and sealing, excessive color of injection, crystallization, etc., to achieve The effect of reducing troubles and improving the yield of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017]According to the dosage of 1000 vials of product specifications of 0.1g / item, the prescription in Table 1 is set as 50mg / ml in terms of lomefloxacin. Now choose glucose as excipient to prepare lomefloxacin aspartate powder, namely: under aseptic conditions, take lomefloxacin aspartate and glucose in the prescription amount in Table 1, place them in a sterilized container, add Appropriate amount of water for injection, stir to dissolve, add water for injection to the full amount, stir well; add 0.02% activated carbon for injection and stir for 5-10 minutes, decarbonize with a sterile filter, filter with a 0.45 μm microporous membrane, and finally 0.22 μm microporous Filter through a pore filter, and after the filtrate is qualified, it is divided into molded bottles, and each bottle with a specification of 0.1g is filled with 2ml, and vacuum freeze-dried: pre-freeze below -30°C for about 2 to 3 hours, and the temperature of the condenser Drop below -45°C → start the vacuum...
Embodiment 2
[0023] According to the dosage of 1000 sticks of product specifications of 0.1 / g stick, the prescription in Table 2 is set as 50 mg / ml based on lomefloxacin. Now select mannitol as excipient to prepare lomefloxacin aspartate powder, that is: under aseptic conditions, weigh lomefloxacin aspartate and mannitol in the prescription amount in Table 2, and place them in a sterile container , add an appropriate amount of water for injection, stir to dissolve, add water for injection to the full amount, and stir well; add 0.02% activated carbon for injection and stir for 5 to 10 minutes, decarbonize with a sterile filter, filter with a 0.45 μm microporous membrane, and finally 0.22 μm microporous membrane filtration, after the filtrate is qualified, it is divided into molded bottles, each bottle with a specification of 0.1g is filled with 2ml, vacuum freeze-drying: pre-freeze below -30°C, and condense after about 2 to 3 hours The temperature of the device drops below -45°C → start the...
Embodiment 3
[0029] 1. Bacterial endotoxin test
[0030] 1) Test product
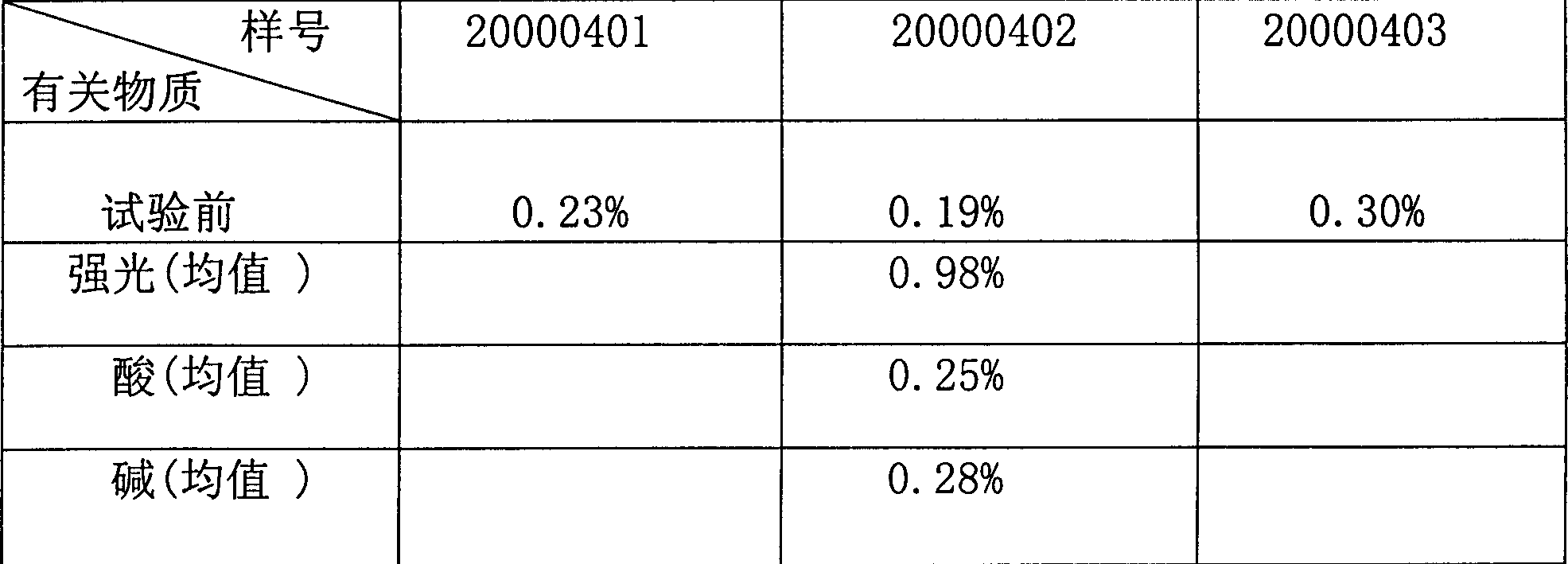
[0031] The pharmaceutical powder of the present invention has sample numbers 20000401, 20000402, and 20000403, and the specification is 0.2g / bottle.
[0032] 2) Reagent
[0033] Limulus reagent 0.1ml / tube, sample number: 0012132, sensitivity 0.125EU / ml, produced by Zhanjiang Andus Biological Co., Ltd.; 0.1ml / tube, sample number: 010701, sensitivity 0.125EU / ml, produced by Fuzhou Xinbei Biochemical Industry Co., Ltd. , and the sensitivity checks are in compliance with the regulations. Bacterial endotoxin test water 2ml / cartridge, sample number 0103060, produced by Zhanjiang Andus Biological Co., Ltd.
[0034] 3) Standard substance:
[0035] Bacterial endotoxin working standard, each containing 70 units, sample number 2001-3, China Institute for the Control of Pharmaceutical and Biological Products.
[0036] 4) Vortex mixer XW-80A, Shanghai Medical University Instrument Factory; bacterial endotoxin tester, Tianji...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com